Arrange the following carbocations according to their stability order
(I)C6H5C+H2
(II) C6H5CH2CH2
(III) C6H5C+HCH3
(IV) C6H5+C(CH3)2
(II)<(I)<(III)<(IV)
(I)<(II)<(III)<(IV)
(III)<(II)<(I)<(IV)
(IV)<(I)<(III)<(I)
The correct Answer is:A
Resonance and inductive effect decide stability of carbocations.
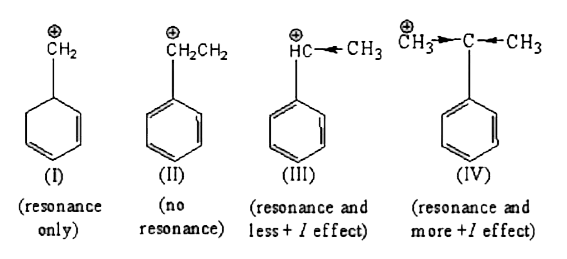
∴ Correct order of stability is
(II)<(I)<(III)<(IV)
Topper's Solved these Questions
Similar Questions
How many lone pairs are present at an angle approximate to 90∘ in CIF3 molecule?
View SolutionWhat is the correct order of increasing C - O bond length of CO, CO2−3 and CO2 ?
View SolutionArrange the following compounds in the order of increasing boiling points :
(i) CH3COC1
(ii) (CH3CO)2O
(iii) CH3CONH2
(iv) CH3COOH.
View SolutionThe decreasing order of acidic character of the following is : (I) CH_3 SH (II) CH_3 OH (III) H_2 O (IV) EtOH .
View SolutionArrange the following carbanions in decreasing order of stability :
(i) CH≡Cϴ
(ii) CH3−C=Cϴ
(iii) CH2=ϴCH
CH3−ϴCH2
View SolutionConsider the following carbocations
(I)C6H5+CH2 , (II)C6H5CH2+CH2
(III)C6H5+CHCH3 , (IV) C6H5+C(CH3)2
The correct sequence for the stability of these is
View SolutionArrange following carbocation in the decreasing order of stability (i) CH_3-overset(+)CH-CH_3 (ii) CH_3-overset(+)CH-O-CH_3 (iii) CH_3-overset(+)(CH)-CO-CH_3
View Solutionनिम्नलिखित कार्बोधनाथनों में,
I.(CH3)2CH+, II. CH3CH+2 , III.C6H5CH+2, IV. (CH3)3C+
स्थायित्व का सही क्रम है
View SolutionWhat is the type of hybridisation of each carbon in the following compounds ?
(i) CH3CH3
(ii) CH3CI
(iii) (CH3)2CO
(iv) CH3CN
View SolutionArrange the following
CH3CH2CH2Cl(I),CH3CH2−CHCl−CH3(II),(CH3)2CHCH2Cl(III)and(CH3)3C−Cl(IV) in order of decreasing tendency towards S2N reaction
View SolutionArrange the following carbocations in the increasing order of their stability. Justify. CH_3^+ , CH_3CH_2^+ , (CH_3)_2CH^+
View SolutionArrange in order of increasing stability : overset(-)(C)H_3,(CH_3)_2overset(-)(C)H,(CH_3)_3overset(-)C,CH_3overset(-)(C)H_2
View SolutionArrange the following in the order as mentioned : overset(+ )CH_3 CH_3 overset(+)CH_2, (CH_3)_2 CHoverset(+), (CH_3)_3 + C carbocations in the order of increasing reactivity,
View SolutionArrange the following carbocation in ascending order of their stabilities (CH_3)_3 overset(oplus)C,overset(oplus)(CH_3),CH_2overset(oplus)CH_2,(CH_3)_2 overset(oplus)CH
View SolutionArrange the following compounds in the order of increasing boiling points : (i) CH_3COC1 (ii) (CH_3 CO)_2 O (iii) CH_3 CONH_2 (iv) CH_3 COOH .
View SolutionThe decreasing order of acidic character of the following is : (I) CH_3 SH (II) CH_3 OH (III) H_2 O (IV) EtOH .
View Solution

