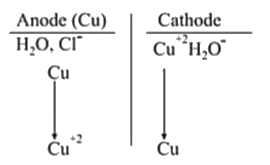
In the electrolysis of CuCl2, solution, the mass of cathode increase by 6.4 g. What occurred at copper anode?
0.224 litre of Cl2 was liberated
1.12 litre of oxygen was liberated
0.05 mole Cu2+ passed into the solution.
0.1 mole Cu2+ passed into the solution
The correct Answer is:D
CuCl2(aq)⇔Cu+2+2Cl−
Topper's Solved these Questions
Similar Questions
In the electrolysis of an aqueous Cr2(SO4)3 solution using a current of 2 amp, the mass of cathode is increased by 8 g. How long was electrolysis conducted ?
View SolutionIn the electrolysis of aqueous solution of CuSO4 using copper electrodes , the process tha takes place at the anode is
View Solution
Knowledge Check
In the electrolysis of CuCl2 solution, the mass of cathode increased by 6.4 g. What occurred at copper anode ?
A0.224 litre of Cl2 was liberatedB1.12 litre of oxygen was liberatedC0.05 mole Cu2+ passed into the solutionD0.1 mole Cu2+ passed into the solutionIn the electrolysis of CuCl2 solution (aq) with Cu electrodes, the weight of cathode increased by 3.2 g. In the anode,
A0.05 mole of Cu2+ will go into the solutionB560 of O2 will be liberatedC112 mL of Cl2 will be liberatedD3.2 mole of Cu2+ will go into the solutionIn electrolysis of aqueous copper sulphate, the gas at anode and cathode is
AO2 and H2BSO2 and H2CH2 and O2DSO3 and O2
In the manufacture of NaOH by the electrolysis of NaCl solution , the cathode and anode are separated using a diaphragm because
View SolutionDuring the electrolysis of fused PbBr2 the reaction that occurs at the cathode is :
View SolutionWhich of the following statements are correct?
a) The electrolysis of aquious NaCl produces hydrogen gas at cathode and chlorine gas at anode,
b) The electrolysis of a CuSO4 solution using Pt electrodes causes the liberation of O2 at the anode and the deposition of copper at the cathode.
c) Oxygen and hydrogen are produced at the anode and cathode during the electrolysis of dilute aqueous solution of H2SO4
d) All electrolytic reactions are redox reactions
View SolutionIn electrolysis of dilute H2SO4 what is liberated at anode?
View SolutionDuring the electrolysis of molten NaCl solution, 230 g of sodium metal is deposited on the cathode, then how many moles of chlorine will be obtained at anode?
View Solution

