Calculate the bond order of C-O bond in CO2−3 ion.
The correct Answer is:1.33
When a single Lewis structure of a molecule cannot describe its all properties, a number of structures, called canonical structers, are written. The actual structure known as resonance hydrid, is in between of all these canonical forms. This phenomenon is called resonance.
According to experiment findings, all carbon to oxygen bonds in CO2−3 are equivalent while in a single Lewis structure, there are two single bonds and one double bond between carbon and oxygen. So, a single Lewis structure is inadequate for the representation of CO2−3 ion. The carbonate ion is best described as hybrid of the canonical or resonance forms I, II and III.
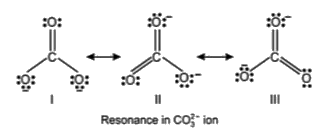
All canonical forms have similar energy, same positions of atoms and same number of bonded and non bonded pairs of electrons.
Average bond order =2+1+13=1.33
Topper's Solved these Questions
Similar Questions
In solid-state PC15 exists in ionic form. Mark the correct hybridization state of Phosphorus in cationic and anionic form respectively.
View SolutionThe count of ligand(s) among the following which are classified as ambidentate ligands:
1. CO
2. CN
3. F
4. OH−
5. SCN
6. NO−2
7. CH3NH2
8. H2N(CH2)2NH2
View SolutionThe bond length of C=O bond in CO is 1.20Å and in CO2 it is 1.34Å Then C=O bond length in CO2−3 will be .
View SolutionThe bond order in CO2−2 ion between C - O is
View SolutionThe correct order of increasig C-O bond length of CO, CO_(3)^(2-), CO_(2) is
View SolutionBond order of N-O bonds in nitrate ion is
View SolutionThe bond order of the N-O bonds in NO−3 ion is
View SolutionThe correct order of increasing C-O bond length of CO, CO_(3)^(2-) , CO_(2) is
View SolutionThe correct order of increasing C-O bond length of CO, CO2−3,CO2 is-
View SolutionExplain the equal bond lengths of C - O bonds in CO_(3)^(2) ion .
View SolutionThe correct order of C−O bond length among CO,CO2−3,CO2 is
View SolutionThe bond order of the N-O bonds in NO_3^- ion is
View SolutionThe correct order of increasing C-O bond strength of CO,CO_(3)^(2-),CO_(2) is :
View SolutionThe C−O bond order in CO and CO2 respectively
View SolutionThe correct order of decreasing C−O bond length of (1) CO,(II)CO2−3(III)CO2 is .
View SolutionWhich is the correct order number for bond length of C -O in CO, CO2−3andCO2 ?
View Solution

