Which of the following options does not represent ground state electronic configuration of an atom?
1s22s22p23s22p63d84s2
1s22s22p63s23p63d94s2
1s22s22p63s23p63d104s1
1s22s22p63s23p63d54s1
The correct Answer is:B
Correct configuration (b) should be 1s22s22p63s23p63d104s1 (for .29Cu)
Topper's Solved these Questions
- STRUCTURE OF ATOMBOOK - PRADEEPCHAPTER - STRUCTURE OF ATOMEXERCISE - NCERT Exemplar Problems With Answers, Hints and Solution (II. Multiple Choice Questions)
5 Videos
- STRUCTURE OF ATOMBOOK - PRADEEPCHAPTER - STRUCTURE OF ATOMEXERCISE - NCERT Exemplar Problems With Answers, Hints and Solution (Short Answer questions)
20 Videos
- STRUCTURE OF ATOMBOOK - PRADEEPCHAPTER - STRUCTURE OF ATOMEXERCISE - NCERT Questions And Exercises with Answers
67 Videos
- STATES OF MATTER: SOLID MATTERBOOK - PRADEEPCHAPTER - STATES OF MATTER: SOLID MATTEREXERCISE - COMPETITION FOCUS (ASSERTION-REASON)
17 Videos
- THERMODYNAMICSBOOK - PRADEEPCHAPTER - THERMODYNAMICSEXERCISE - MULTIPLE CHOICE QUESTION ( BASED ON PRACTICAL CHEMISTRY)
3 Videos
Similar Questions
Identify the paris which are not of isotopes?
View SolutionArrange s,p and d subshells of a shell in the increasing order of effective nuclear charge (Zeff) experienced by the electron present in them.
View Solution(i) Calculate the number of electrons which will together with one gram .
(ii) Calculate the mass and charge on one mole of electrons .
View SolutionStatement -1 : Covalent crystals have the highest melting point .
Statement 2: Covalent bonds are stronge than ionic bonds.
View Solution50mL of 0.10 B Ba(OH)2 is added to 50 mLof 0.10MH2SO4. The rise in temperature isΔT1 . If experiment is replaced by taking 100 mL of each solution, the rise in temperature isΔT92). Then
View SolutionWhich of the following options does not represent ground state electronic configuration of an atom?
View SolutionGround state electronic configuration of nitrogen atom can be represented by:
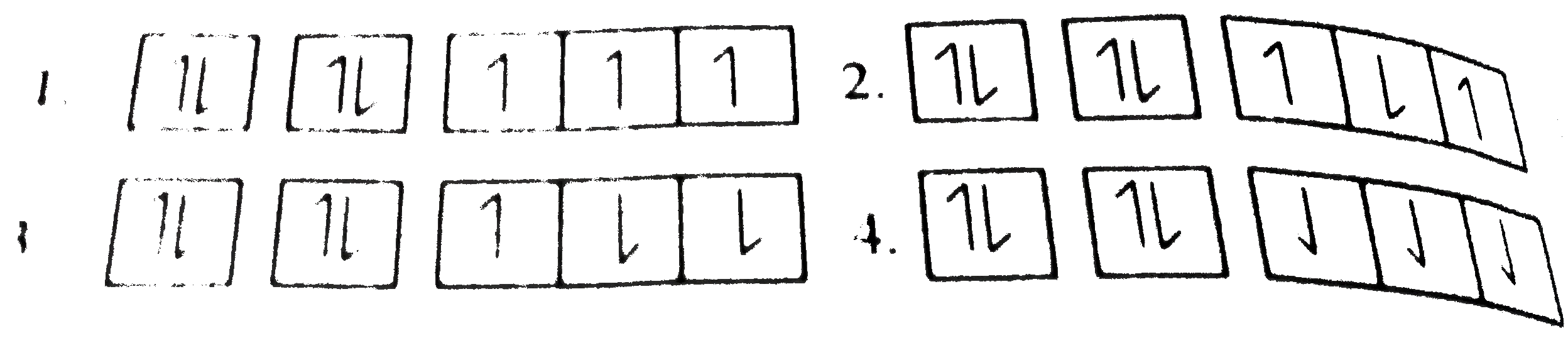
View SolutionThe ground state electronic configuration of nitrogen atom can be represented as:
View SolutionGround state electronic configuration of nitrogen atom can be represented by
View SolutionGround state electronic configuration of nitrogen atom can be represented by:
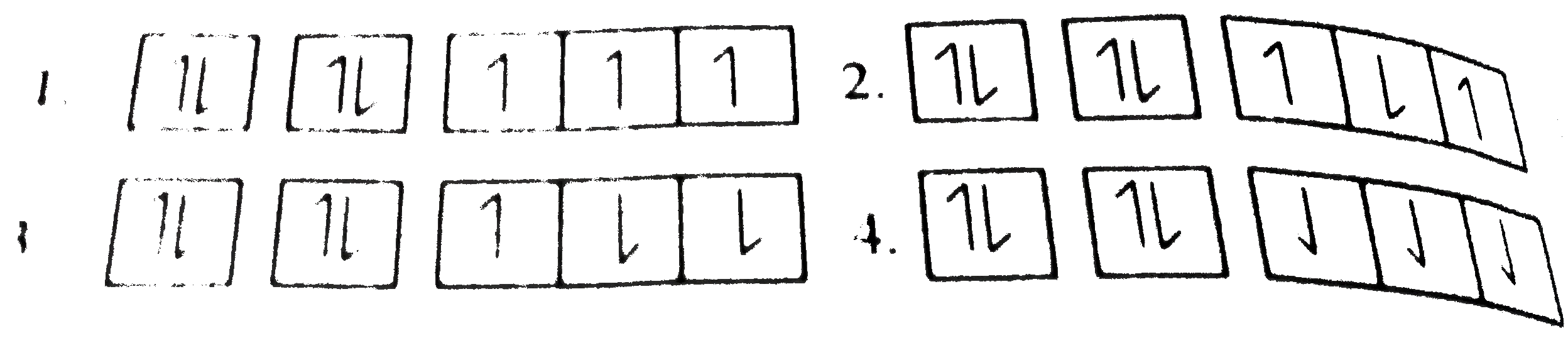
View SolutionWhich of the following options does not represent ground state electronic configuration of an atom?
View SolutionWhich of the following options dose not represent electronic ground state of an atom ?
View SolutionWhich of the following do not represent ground state electronic configuration of an atom ?
View SolutionGround state electronic configuration of p atom can be represented as
View SolutionWhich of the following options does not represent ground state electronic configuration of an atom?
View Solution

