Products of the following reaction Me2C=CHCH3(i)O3−−−−−−−−→(ii)(CH3)2S? Are
CH3CHO+CH3COOH
Me2CO+CH3CHO
Me2CO+CH3COOH
2Me2CO
The correct Answer is:B
To solve the question regarding the products of the reaction of the alkene Me2C=CHCH3 with ozone followed by dimethyl sulfide, we can break down the process into clear steps:
Step 1: Identify the Alkene
The given alkene is Me2C=CHCH3, which can be represented as:
- A carbon-carbon double bond between the second and third carbon atoms.
- The structure can be written as:
CH3∣CH3−C=C−CH3
Step 2: Ozonolysis Reaction
When the alkene undergoes ozonolysis (reaction with ozone), it forms an ozonide. The double bond is cleaved, and ozone adds across the double bond.
- The ozonide formation will result in:
- One carbon from the double bond will be attached to two methyl groups (Me2C).
- The other carbon will be attached to one methyl group and one hydrogen (CH3-CH).
The ozonide structure can be represented as:
OCH3−CC−CH3 O
Step 3: Reduction of Ozonide
The ozonide is then treated with dimethyl sulfide ((CH3)2S), which reduces the ozonide to yield carbonyl compounds (aldehydes and ketones).
- The cleavage of the ozonide will yield:
- A ketone from the carbon with two methyl groups (Me2C) which will form acetone (CH3COCH3).
- An aldehyde from the carbon with one methyl group and one hydrogen which will form ethanol (CH3CHO).
Step 4: Write the Final Products
The final products of the reaction can be summarized as:
- Acetone (CH3COCH3)
- Ethanal (CH3CHO)
Thus, the products of the reaction are:
1. Acetone (CH3COCH3)
2. Ethanal (CH3CHO)
Final Answer
The products of the reaction Me2C=CHCH3 with ozone followed by dimethyl sulfide are:
- Acetone (CH3COCH3)
- Ethanal (CH3CHO)
---
The given alkene is Me2C=CHCH3, which can be represented as:
- A carbon-carbon double bond between the second and third carbon atoms.
- The structure can be written as:
CH3∣CH3−C=C−CH3
When the alkene undergoes ozonolysis (reaction with ozone), it forms an ozonide. The double bond is cleaved, and ozone adds across the double bond.
- One carbon from the double bond will be attached to two methyl groups (Me2C).
- The other carbon will be attached to one methyl group and one hydrogen (CH3-CH).
OCH3−CC−CH3 O
The ozonide is then treated with dimethyl sulfide ((CH3)2S), which reduces the ozonide to yield carbonyl compounds (aldehydes and ketones).
- A ketone from the carbon with two methyl groups (Me2C) which will form acetone (CH3COCH3).
- An aldehyde from the carbon with one methyl group and one hydrogen which will form ethanol (CH3CHO).
The final products of the reaction can be summarized as:
- Acetone (CH3COCH3)
- Ethanal (CH3CHO)
1. Acetone (CH3COCH3)
2. Ethanal (CH3CHO)
The products of the reaction Me2C=CHCH3 with ozone followed by dimethyl sulfide are:
- Acetone (CH3COCH3)
- Ethanal (CH3CHO)
Topper's Solved these Questions
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:Competition Focus (JEE(main and advanced)/Medical Entrance) II. MULTIPLE CHOICE
Explore 13 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:Competition Focus (JEE(main and advanced)/Medical Entrance) III. MULTIPLE CHOICE
Explore 14 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)
Explore 2 Videos
- EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESBook:PRADEEPChapter:EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESExercise:Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)
Explore 10 Videos
- HYDROGEN Book:PRADEEPChapter:HYDROGEN Exercise:COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
Explore 15 Videos
Similar Questions
In the Newman projection for 2,2-dimethylbutane
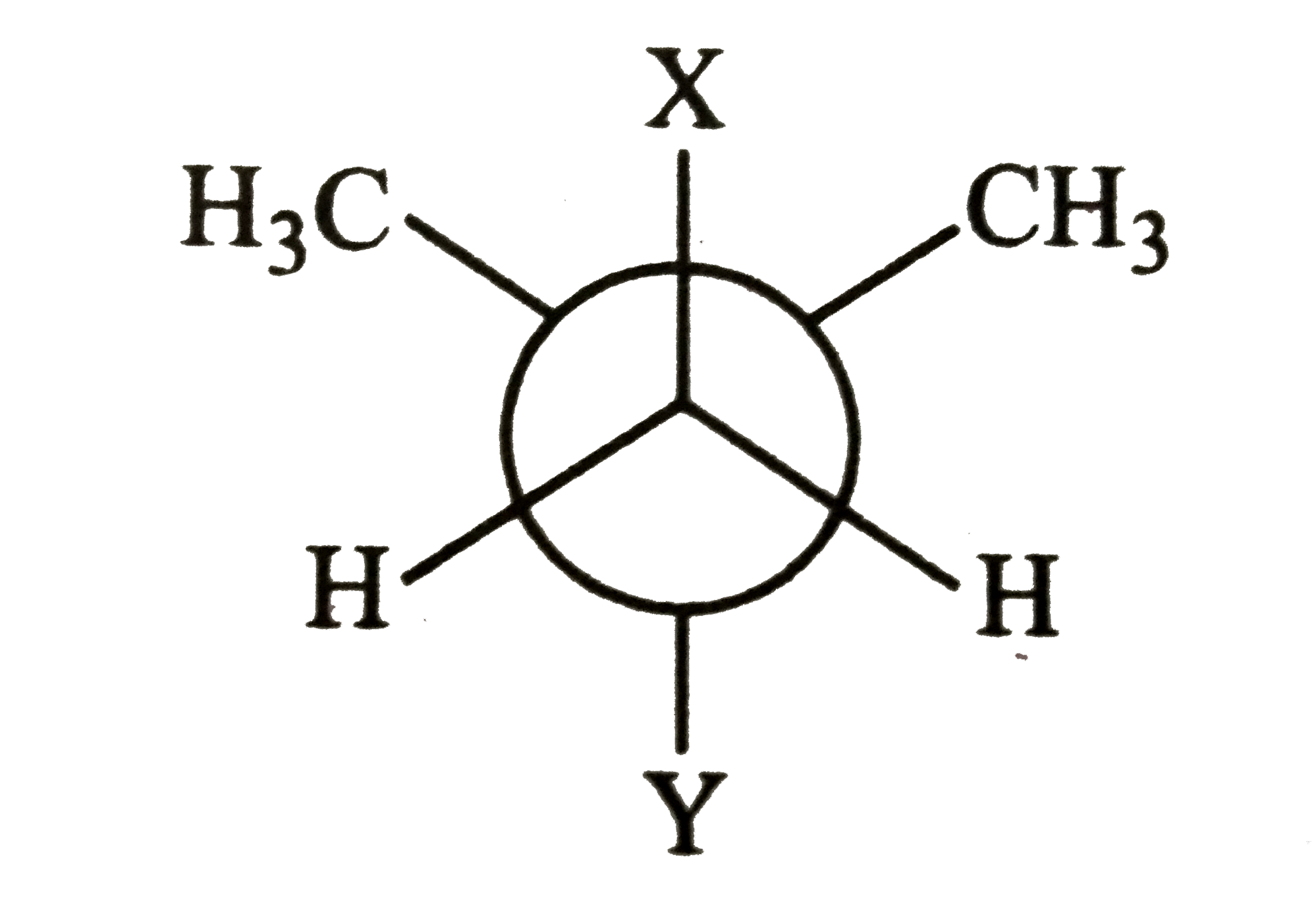
X and Y can respectively be
View SolutionCatalytic hydrogenation involves addition of hydrogen to a gt C = Clt or -C ≡ C- bond in presence of a metal catalyst such as Ni, Pt, Pd, etc. These are called heterogeneous catalysts. Both H2 and alkene/alkyne get adsorbed on the surface of the metal , and syn-addition of hydrogen atoms takes place. The relative rates of hydrogenation follows the order:
−C≡C−>>C=C<>C=o<C6H6
If sodium or lithium in liquid ammonia is used, anti-addition of H-atoms occurs across −C≡C−bond. These reducing agents can also be used to reduce double bonds of benzene rings and conjugated dienes but not of isolated dienes.
Which of the following alkenes will react most readily with H2 under catalytic hydrogeneration conditions ?
View SolutionTwo isomeric compounds X and Y have the molecular formula C7H7OH. X gives purple colour with FeCl3 while Y does not. What are X and Y ?
View SolutionIn each of the following questions, a statement of Assertion is given followed by a corresponding statement of Reason just below it. Of the statements, mark the correct answer as
Assertion . The vapour pressure of a pure liquid has a fixed value at a particular temperature .
Reason . When equilibrium is reached , no more vapour are formed .
View SolutionAssertion : The water gas shift reaction can be used to increase the amount of H2 in the shift ' syn gas ' mixture.
Reason : In this reaction, water is reduced to H2 by CO.
View Solutionनिम्नलिखित अभिक्रिया में उत्पाद है:
CH3−CH3∣∣C∣CH3−CH=CH2H2∅H+→
View SolutionCH3−CH3∣C=O(i)CH3MgI−−−−−−−→(ii)H2OX
The product obtained in this reaction is ________.
View SolutionWrite IUPAC names of the following compounds : (i) CH_3-C(CH_3)-underset(CH(CH_3)_2)underset(|)C=CHCH_3
View SolutionWhat will be compound A in the following reaction- H_3C-overset(CH_3)overset(|)underset(CH_3)underset(|)C-CH=CH_2overset((i)Hg(OOC""CH_3)_2,THF)underset((ii)NaBH_4,NaOH)toA
View SolutionA1.O3−−−−−−→2.Zn,H2O(CH3)2CO+HCHO+CHO∣∣∣CHO Thus A is :
View SolutionThe major products of the following reaction are :
CH3−CH3∣CH−CH−CH3∣OSO2CH3(i)KOtBu/Δ−−−−−−−−→(ii)O3/H2O2
View SolutionThe product Z in the following series of reaction is : CH_3CH_2 -overset(CH_3)overset(|)C=CH_2 underset((ii)NaBH_4)overset((i)Hg(OAc)_2)toY underset((ii)CH_3Br)overset((i)Na)to Z
View Solutionनिम्न क्रिया का उत्पाद होगा
CH3−CH3∣C∣CH3−CH=CH2(I)Hg(Oac)2H2O−−−−−−−−−−−→(ii)NaBH4
View Solutionनिम्नलिखित अभिक्रिया का उत्पाद है
CH3C≡CCH2CH3(1)O3−−−−−−→(2) जलअपघटन
View SolutionThe major products of the following reaction are :
CH3−CH3∣CH−CH−CH3∣OSO2CH3(i)KOtBu/Δ−−−−−−−−→(ii)O3/H2O2
View Solution
