One mole of a symmetrical alkene on ozonolysis gives two moles of an aldehyde having a molecular mass of 44u. The alkene is:
ethene
propene
1-butene
2-butene
The correct Answer is:D
The aldehyde with molecular mass 44 u is CH3CHO (acetaldehyde). Therefore, the symmetrical alkene is 2-butene
CH3CH=CHCH32-Butene(i)O3/CH2Cl2,196K−−−−−−−−−−−−−→(ii)Zn/H2O2CH3CHOAcetaldehyde(Mol. mass =44 u)
Topper's Solved these Questions
- HYDROCARBONSBOOK - PRADEEPCHAPTER - HYDROCARBONSEXERCISE - Competition Focus (JEE(main and advanced)/Medical Entrance) II. MULTIPLE CHOICE
13 Videos
- HYDROCARBONSBOOK - PRADEEPCHAPTER - HYDROCARBONSEXERCISE - Competition Focus (JEE(main and advanced)/Medical Entrance) III. MULTIPLE CHOICE
14 Videos
- HYDROCARBONSBOOK - PRADEEPCHAPTER - HYDROCARBONSEXERCISE - ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)
2 Videos
- EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESBOOK - PRADEEPCHAPTER - EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESEXERCISE - Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)
10 Videos
- HYDROGEN BOOK - PRADEEPCHAPTER - HYDROGEN EXERCISE - COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
15 Videos
Similar Questions
In the Newman projection for 2,2-dimethylbutane
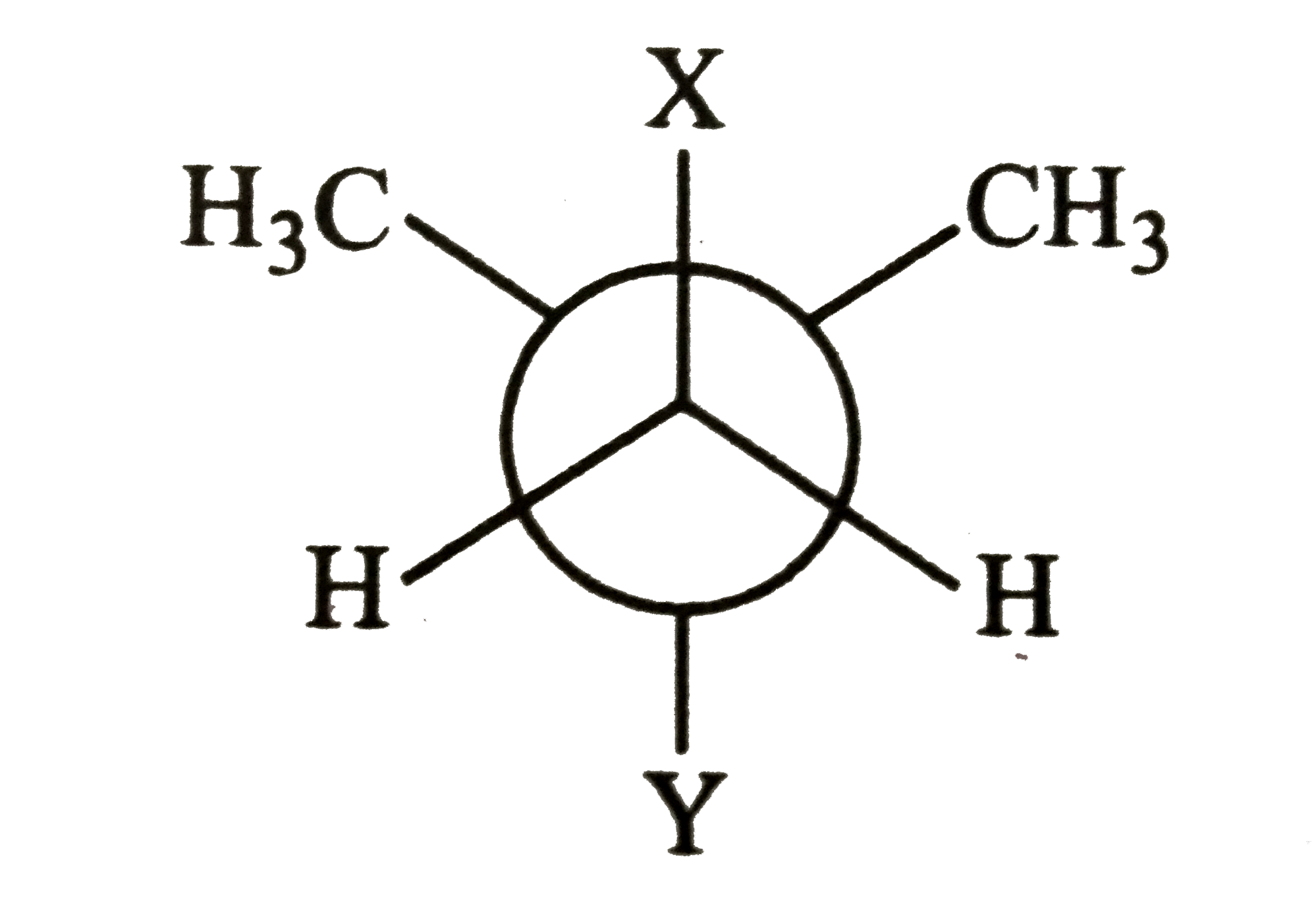
X and Y can respectively be
View SolutionCatalytic hydrogenation involves addition of hydrogen to a gt C = Clt or -C ≡ C- bond in presence of a metal catalyst such as Ni, Pt, Pd, etc. These are called heterogeneous catalysts. Both H2 and alkene/alkyne get adsorbed on the surface of the metal , and syn-addition of hydrogen atoms takes place. The relative rates of hydrogenation follows the order:
−C≡C−>>C=C<>C=o<C6H6
If sodium or lithium in liquid ammonia is used, anti-addition of H-atoms occurs across −C≡C−bond. These reducing agents can also be used to reduce double bonds of benzene rings and conjugated dienes but not of isolated dienes.
Which of the following alkenes will react most readily with H2 under catalytic hydrogeneration conditions ?
View SolutionTwo isomeric compounds X and Y have the molecular formula C7H7OH. X gives purple colour with FeCl3 while Y does not. What are X and Y ?
View SolutionIn each of the following questions, a statement of Assertion is given followed by a corresponding statement of Reason just below it. Of the statements, mark the correct answer as
Assertion . The vapour pressure of a pure liquid has a fixed value at a particular temperature .
Reason . When equilibrium is reached , no more vapour are formed .
View SolutionAssertion : The water gas shift reaction can be used to increase the amount of H2 in the shift ' syn gas ' mixture.
Reason : In this reaction, water is reduced to H2 by CO.
View SolutionOne mole of symmetrical alkene on ozonolysis gives two moles of an aldehyde having a molecular mass 58. The alkene
View SolutionAn alkene gives two moles of HCHO , one mole of CO_(2) and one mole of CH_(3)COCHO on ozonolysis. The structure of alkene is
View SolutionOne mole of symmetrical alkene upon ozonolysis gives two moles of an aldehyde having the molecular mass 44u. Predict the alkene.
View SolutionOne mole of alkene an ozonolysis gives two moles of butanone. The alkene is :
View SolutionOne mole of a symmetrical alkene on hydrolyses gives two moles of an aldehyde having a molecular mas of 44 u. The alkene is
View SolutionOne mole of alkene on ozonolysis gives 2 moles of butanone. The alkene is:
View SolutionOne mole of a symmetrical alkene on ozonolysis gives two of an aldehyde having a molecular mass of 44u. The alkene is :
View SolutionOne mole of alkene on ozonolysis gives of butanone. The alkene is
View Solutionएक मोल समपित ऐल्कीन आओजोनीकरण पर दो मोल एल्डिहाइड देता है जिसका आण्विक द्रव्यमान 44 u है। ऐल्कीन है :
View Solutionएक सममित ऐल्कीन के एक मोल का ओजोनीकरण करने पर एक ऐल्डिहाइड के दो मोल प्राप्त होते हैं जिनका अणुभार 44u है। यह ऐल्कीन है:
View SolutionOne mole of a symmetrical alkene on ozonolysis gives two moles of an aldehyde having a molecular mass of 44u. Identify the alkene.
View Solution

