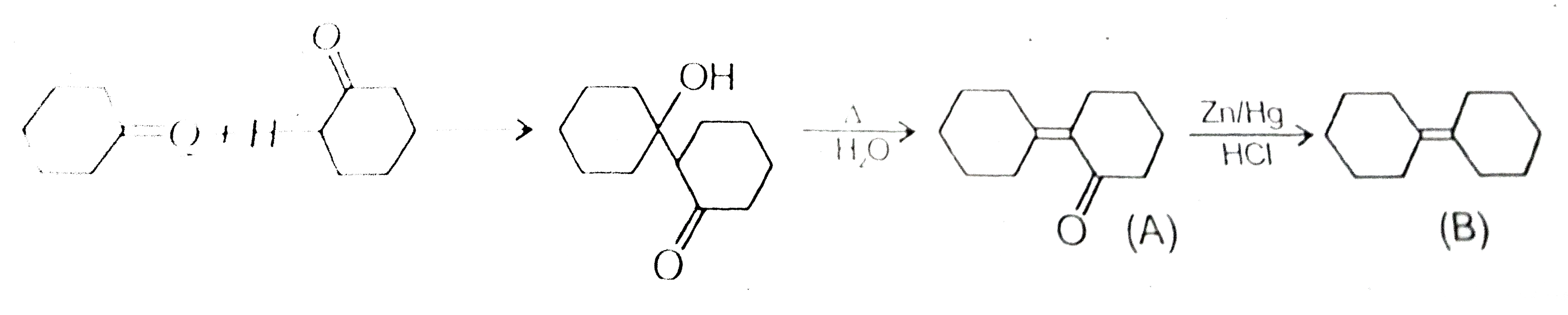Complete the reaction

Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS BOOK - AAKASH INSTITUTECHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDS EXERCISE - Assignment (SECTION -J Akash challengers Questions)
7 Videos
- ALCOHOLS, PHENOLS AND ETHERS BOOK - AAKASH INSTITUTECHAPTER - ALCOHOLS, PHENOLS AND ETHERS EXERCISE - Try Yourself
5 Videos
- AMINES BOOK - AAKASH INSTITUTECHAPTER - AMINES EXERCISE - Assignment Section -J (Aakash Challengers Questions)
4 Videos
Similar Questions
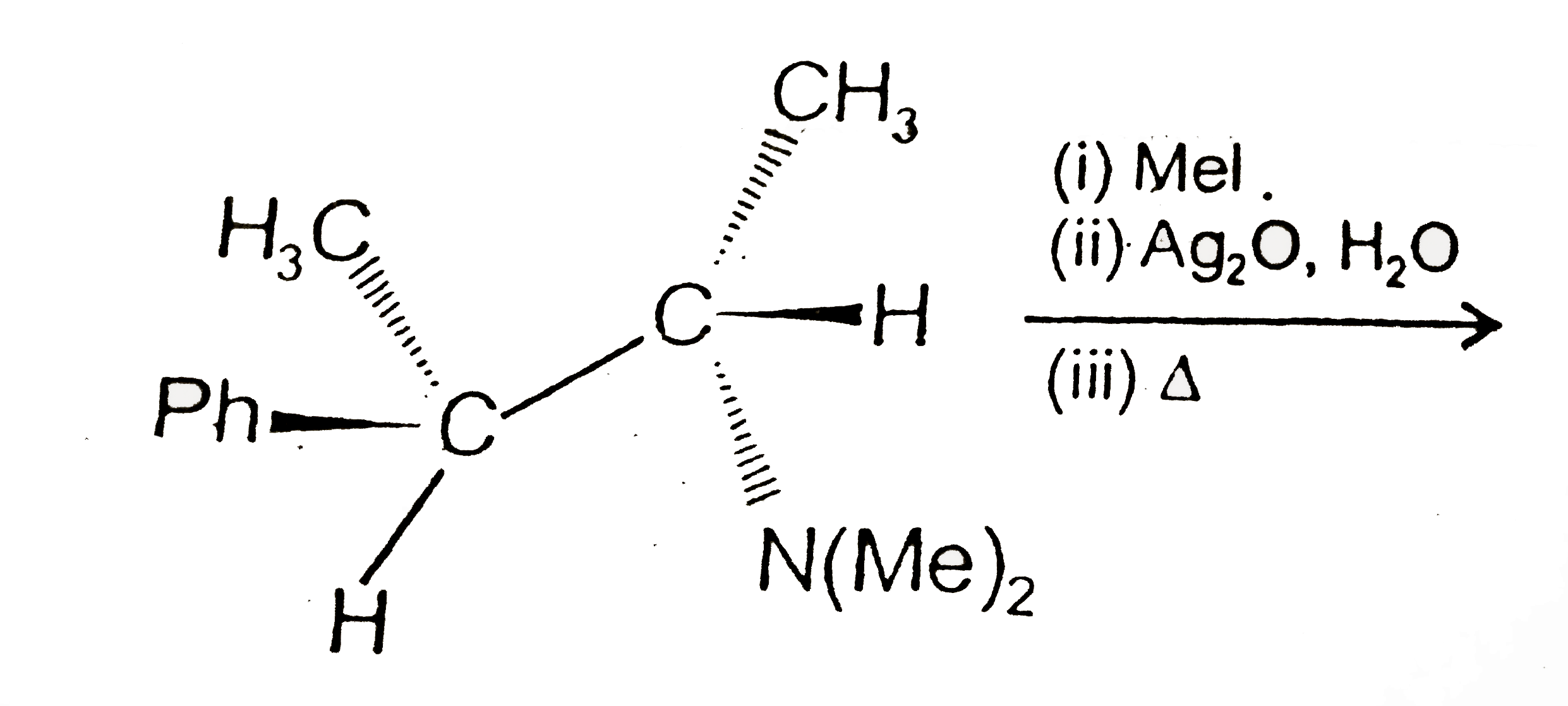
Consider the following sequence of reactions:
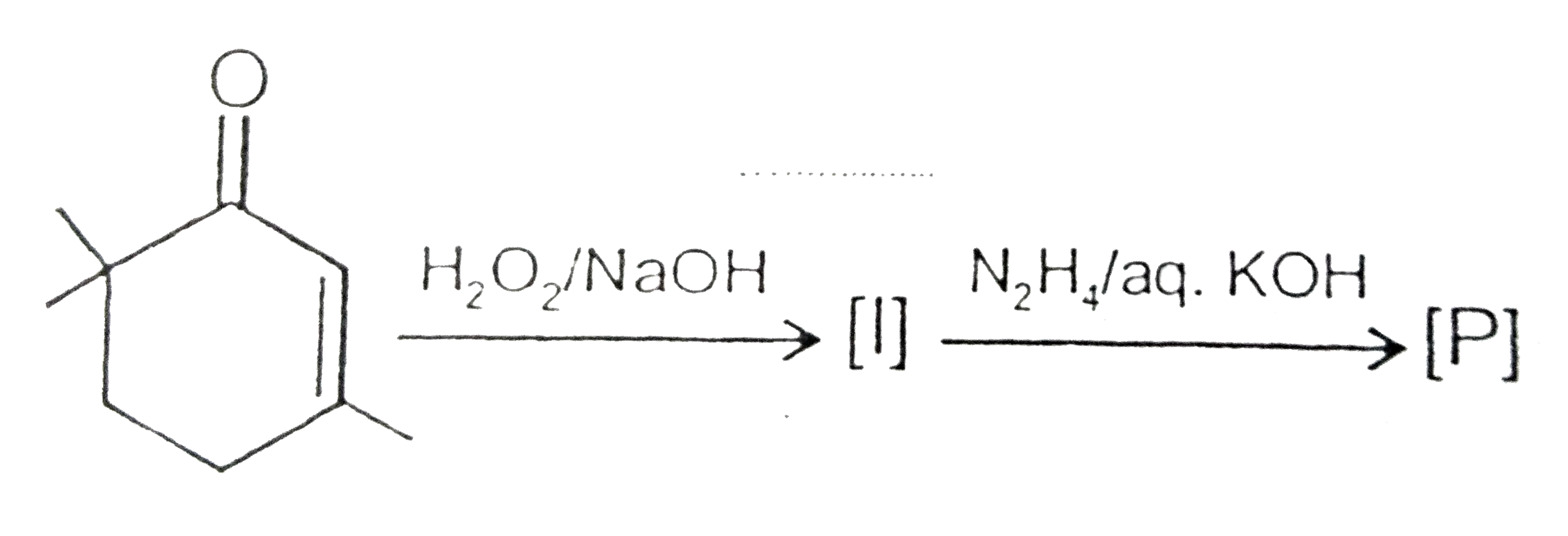
Intermediate [I] and corresponding major product [P] in the given reactions are
View SolutionFind the product of given reaction
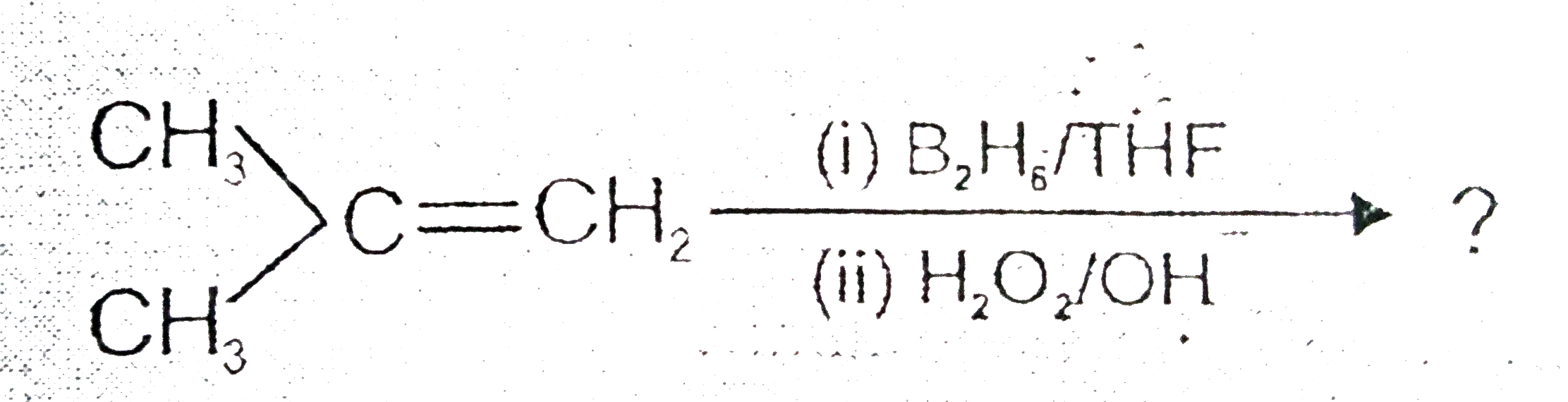
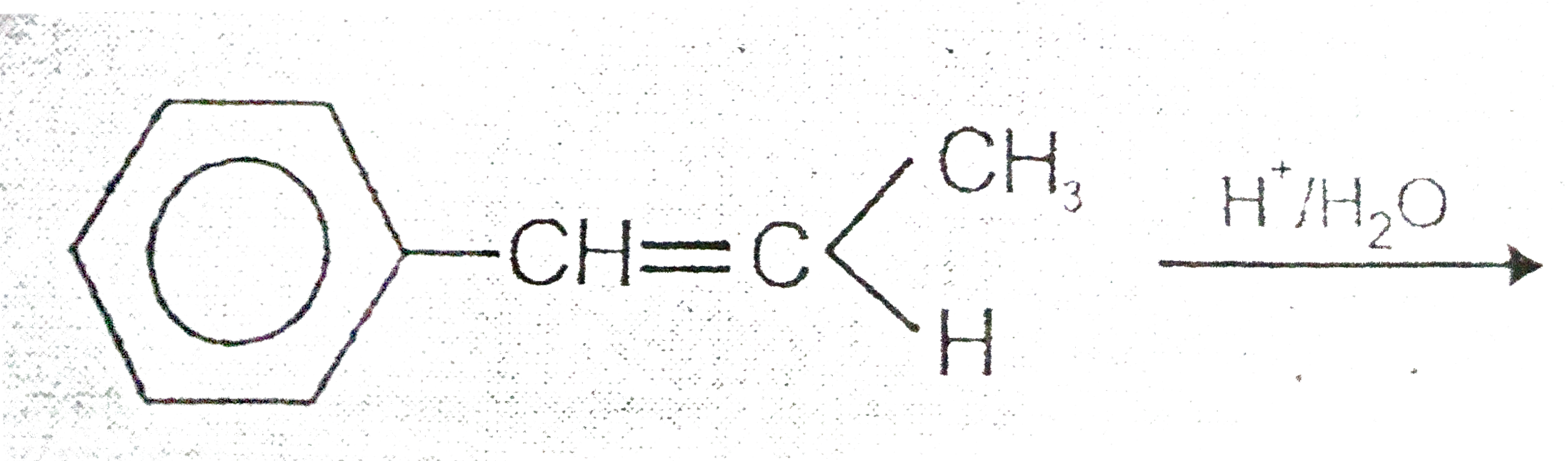

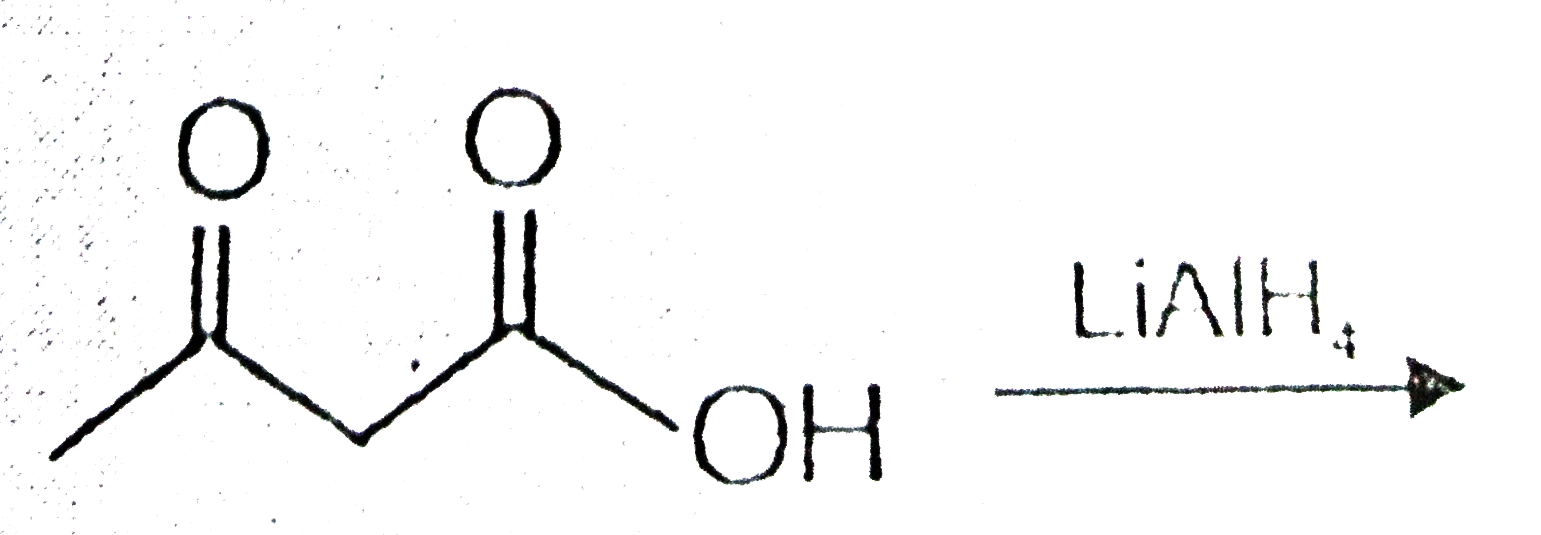
View SolutionThe major product obtained in the reaction is
View SolutionFor the first order reaction, calculate the ratio of the time taken to complete 99% of the reaction to the time taken to complete 90% of the reaction.
View SolutionThe ratio of the times of 99.9 % of the reaction to complete and half of the reaction to complete is
View SolutionIf 75% of first order reaction is completed in 32 min, then 50% of the reaction would be complete in:
View SolutionFor the reaction : A→ Products, the time for 75% completion of reaction is 5 times for 50% completion of reaction. The order of reaction is :
View Solution75% of a first order reaction complete in 4h . 87.5% of the same reaction completes in-
View Solution75% of the first order reaction completed in 3 min. 50% of the reaction was completed in
View SolutionFor a first order reaction, if the time taken for completion of 50% of the reaction is t second, the time required for completion of 99.9% of the reaction is:
View SolutionIn the first order reaction 10% of the reaction is completed in 10 hours. The percentage of the reaction completed in 20 hours is
View SolutionComplete the reaction as follows.
Complete oxidation of methane :
View SolutionComplete the reaction as follows.
Complete oxidation of butane :
View SolutionComplete the reaction as follows.
Complete oxidation of alkane :
View Solution…………….. Reactions never go to completion whereas ……………. Reactions proceed almost to completion.
View SolutionIn a zero order reaction , 20% of the reaction complete in 10 s. How much time it will take to complete 50% of the reaction ?
View Solution