For AB bond if percent ionic character is plotted
against electronegativity difference (XA−XB), the
shape of the curve would look like
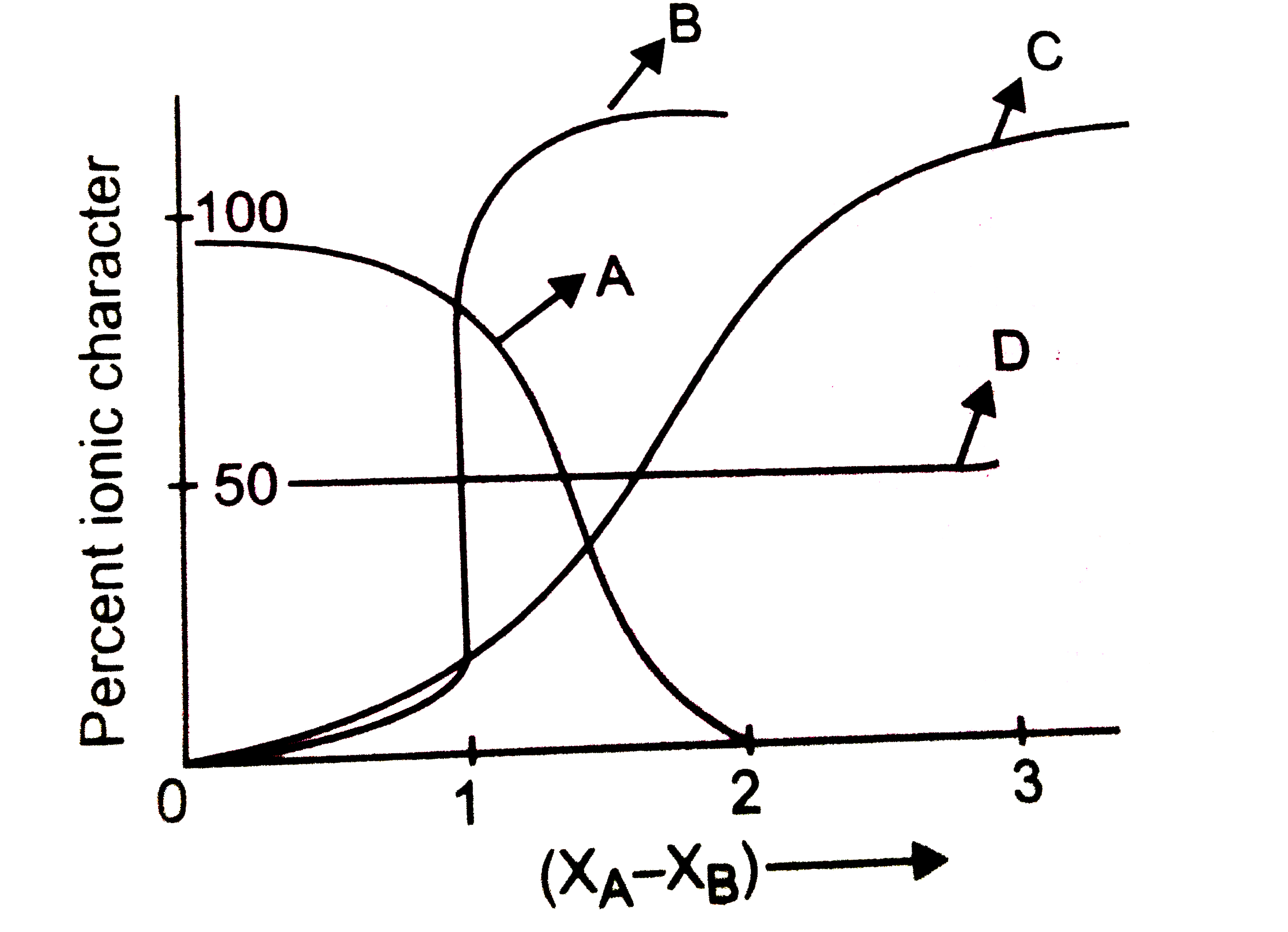
A
B
C
D
The correct Answer is:C
Ionic character is 50% when electronegativity
difference is about 1.9 which is for curve C.
Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:PRADEEPChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (II. MULTIPLE CHOICE QUESTINS (WITH ONE OR MORE THAN ONE CORRECT ANSWER))
Explore 13 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:PRADEEPChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (III. MULTIPLE CHOICE QUESTIONS (Based on the given Passage/Comprehension))
Explore 11 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:PRADEEPChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)
Explore 3 Videos
- APPENDIXBook:PRADEEPChapter:APPENDIXExercise:MODEL TEST PAPER <br> (Section C )
Explore 9 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Book:PRADEEPChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Exercise:Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)
Explore 12 Videos
Similar Questions
Which of the following statements are not correct ?
View SolutionThe study of diple moment of a molecule is useful to explain the shape of a molecule and also to predict a The net dipole moment of a polyatomic molecule is the resltant of the different bond moments present in that molecule . The values are generalluy expressed in Debye or in the S.I. units in terms of Coulomb meter (C m) 1 Debye is equivalent to
View SolutionThe dipole momnet of KCI is 3.336×10−29Cm which indicates that it is a highly polar molecules. The inter atomic distance between K⊕ and CIΘ in this molecules is 2.6×10−10 m Calculate the dipole moment of KCI molecule if there were opposite charges of one fundamental unit located at each nucleus Calculate the ionic character percentage of KCI .
View SolutionCalcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction:
CaCO3(s)+2HCl(aq)→CaCl2(aq)+CO2(g)+H2O(l)
What mass of CaCO3 is required to react completely with 25mL of 0.75MHCl?
View SolutionIn each of the following questions a Statement of Assertion (A ) is given followed by a corresponding statement of Reason (R ) Just below it . Of the statement mark the correct answer as
Assertion .Helium and beryllim have similar outer electronic configuration of the type ns2
Reason . both are chemcially inert .
View SolutionA Ketelaar triangle showing a plot of average electrongativity (χmean) against electronegativity difference (Δχ)can be used to classify the bond type for binary compound. Which of the following compounds has highest percentage of ionic character ?
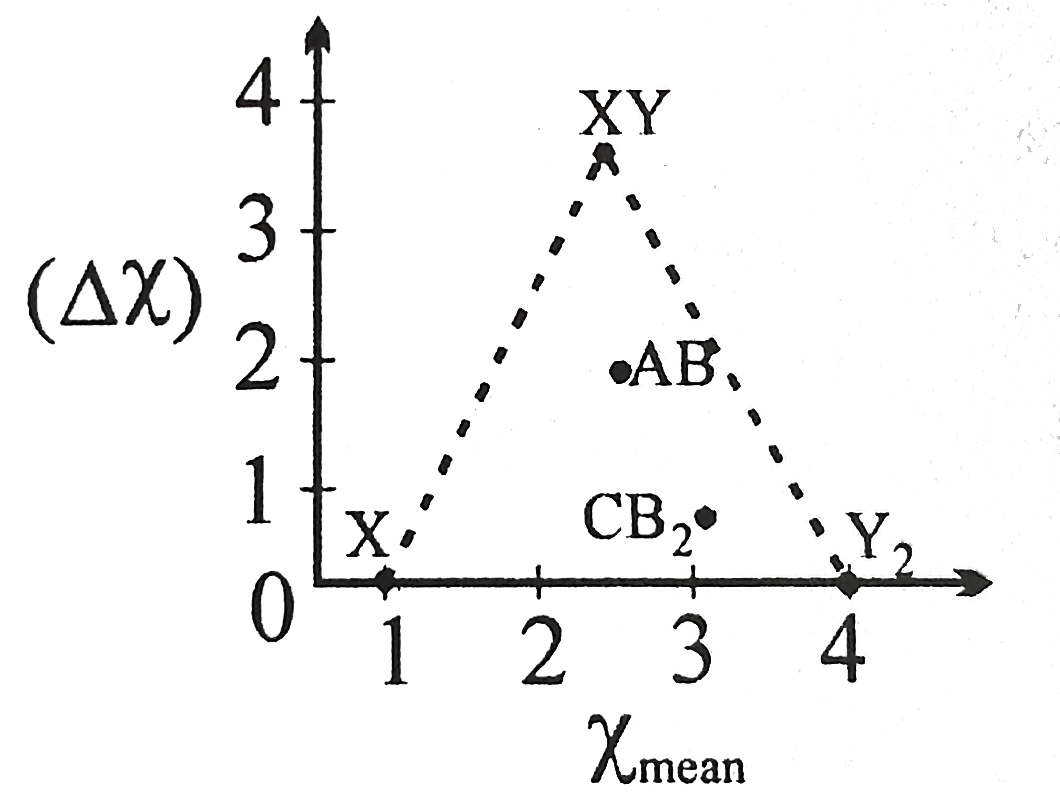
View SolutionNature of bond can be predicted on the basis of electronativity of bonded atoms, greater difference in electronegativity (X), more will be the polarity of bond, and polar bond are easily broken in polar solvent like water. For hydroxy acids X0−XA difference predict the nature of oxide formed by the element A.
|X0−XA|>|X0−XH| then A−O−H show basic nature (NaOH)
|X0−XA|<|X0−XH| then A−O−H show acidic nature (H−O−CI)
With the help of EN values [ENA=1.8,ENB=2.6,ENC=1.6,END=2.8] answer the following questions for the compounds HAO,HBO,HCO,HDO.
Percentage ionic character of compound AB is
View SolutionPauling's equation for determining the electronegativity of an element, is ( X_(A), X_(B) are electronegativity value of elements A and B respectively, Delta represents the polarity of A-B bond.)
View Solution% Ionic character in the covalent bond A-B is: (Given that X_(A) = 2, X_(B) =3)
View SolutionThe percentage ionic character of a covalent bond (A-B) is about 50%, the electronegativity difference of the two elements will be :
View SolutionA polar covalent bond is formed between the two elements of different electronegativies. The polarity of a bond depends on the electronegativity difference, the boiling atoms and also on the shape of the molecule.
A-B Let xA and xB are the electronegativites of bonding atoms A and B then, percentage ionic character of the bond can be calculated, as
% ionic character =21|xA−xB|+3.5|xA−xB|2 Dipole moment of the bond (μ) depends on the bond length and the polarity of the bond.
μ=q×d
Here, q = charge of the dipoles
d = bond length
It is observed that the dipole moment increases with increase in the inductive effect. Answer the following questions as indicated: Select code (a) if the statement is true and code (b) if the statement is false.
The dipole moment of CH3−X bond lies in the following sequence:
CH3−I<CH3−Br<CH3−CI
True (a) False (b)
View SolutionA polar covalent bond is formed between the two elements of different electronegativies. The polarity of a bond depends on the electronegativity difference, the boiling atoms and also on the shape of the molecule. A-B Let x_(A) and x_(B) are the electronegativites of bonding atoms A and B then, percentage ionic character of the bond can be calculated, as % ionic character = 21|x_(A) -x_(B)| +3.5|x_(A)-x_(B)|^(2) Dipole moment of the bond (mu) depends on the bond length and the polarity of the bond. mu = q xx d Here, q = charge of the dipoles d = bond length It is observed that the dipole moment increases with increase in the inductive effect. Answer the following questions as indicated: Select code (a) if the statement is true and code (b) if the statement is false. Dipole moment of methanol is less than ethanol: True (a) False (b)
View SolutionA polar covalent bond is formed between the two elements of different electronegativies. The polarity of a bond depends on the electronegativity difference, the boiling atoms and also on the shape of the molecule.
A-B Let xA and xB are the electronegativites of bonding atoms A and B then, percentage ionic character of the bond can be calculated, as
% ionic character =21|xA−xB|+3.5|xA−xB|2 Dipole moment of the bond (μ) depends on the bond length and the polarity of the bond.
μ=q×d
Here, q = charge of the dipoles
d = bond length
It is observed that the dipole moment increases with increase in the inductive effect. Answer the following questions as indicated: Select code (a) if the statement is true and code (b) if the statement is false.
Dipole moment of C-Cl bond is greater than C-F bond:
True (a) False(b)
View SolutionA polar covalent bond is formed between the two elements of different electronegativies. The polarity of a bond depends on the electronegativity difference, the boiling atoms and also on the shape of the molecule. A-B Let x_(A) and x_(B) are the electronegativites of bonding atoms A and B then, percentage ionic character of the bond can be calculated, as % ionic character = 21|x_(A) -x_(B)| +3.5|x_(A)-x_(B)|^(2) Dipole moment of the bond (mu) depends on the bond length and the polarity of the bond. mu = q xx d Here, q = charge of the dipoles d = bond length It is observed that the dipole moment increases with increase in the inductive effect. Answer the following questions as indicated: Select code (a) if the statement is true and code (b) if the statement is false. Dipole moment of methyl alcohol is less than that of water: True (a) False (b)
View SolutionA polar covalent bond is formed between the two elements of different electronegativies. The polarity of a bond depends on the electronegativity difference, the boiling atoms and also on the shape of the molecule. A-B Let x_(A) and x_(B) are the electronegativites of bonding atoms A and B then, percentage ionic character of the bond can be calculated, as % ionic character = 21|x_(A) -x_(B)| +3.5|x_(A)-x_(B)|^(2) Dipole moment of the bond (mu) depends on the bond length and the polarity of the bond. mu = q xx d Here, q = charge of the dipoles d = bond length It is observed that the dipole moment increases with increase in the inductive effect. Answer the following questions as indicated: Select code (a) if the statement is true and code (b) if the statement is false. Dipole moment of trans-2 butenal is greater than butanal: True (a) False(b)
View SolutionA polar covalent bond is formed between the two elements of different electronegativies. The polarity of a bond depends on the electronegativity difference, the boiling atoms and also on the shape of the molecule. A-B Let x_(A) and x_(B) are the electronegativites of bonding atoms A and B then, percentage ionic character of the bond can be calculated, as % ionic character = 21|x_(A) -x_(B)| +3.5|x_(A)-x_(B)|^(2) Dipole moment of the bond (mu) depends on the bond length and the polarity of the bond. mu = q xx d Here, q = charge of the dipoles d = bond length It is observed that the dipole moment increases with increase in the inductive effect. Answer the following questions as indicated: Select code (a) if the statement is true and code (b) if the statement is false. Dipole moment of para-nitrophenol is greater than phenol: True (a) False(b)
View Solution
