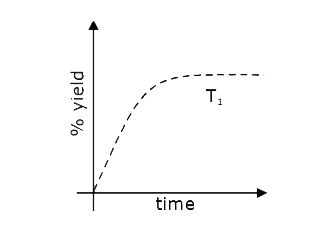
The % yield of ammonia as a function of time in the reaction, N2(g)+3H2(g)⇔2NH3(g)'ΔH<0
at (p,T1) is given below
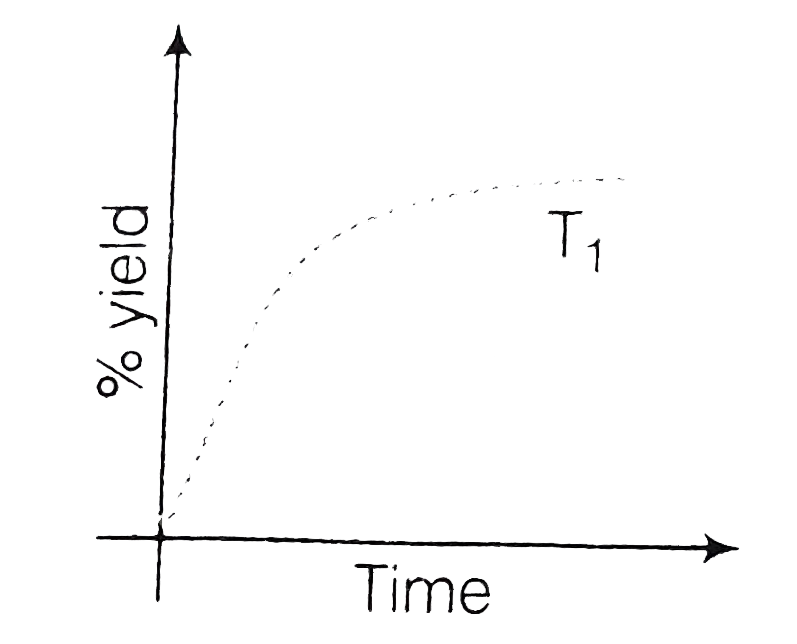
If this reaction is conducted at (p,T1), with T2>T1 the % yield by of ammonia as a function of time is represented by




Step by step video & image solution for The % yield of ammonia as a function of time in the reaction, N_(2)(g)+3H_(2)(g)hArr2NH_(3)(g)'DeltaHlt0 at (p,T_(1)) is given below If this reaction is conducted at (p,T_(1)), with T_(2)gtT_(1) the % yield by of ammonia as a function of time is represented by by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- JEE ADVANCEDBOOK - JEE ADVANCED PREVIOUS YEARCHAPTER - JEE ADVANCEDEXERCISE - Section-3
2 Videos
- JEE ADVANCEDBOOK - JEE ADVANCED PREVIOUS YEARCHAPTER - JEE ADVANCEDEXERCISE - SECTION I
8 Videos
- JEE ADVANCEDBOOK - JEE ADVANCED PREVIOUS YEARCHAPTER - JEE ADVANCEDEXERCISE - Section-1
8 Videos
- JEE (ADVANCED ) 2020BOOK - JEE ADVANCED PREVIOUS YEARCHAPTER - JEE (ADVANCED ) 2020EXERCISE - SECTION -III
6 Videos
- JEE ADVANCED 2020BOOK - JEE ADVANCED PREVIOUS YEARCHAPTER - JEE ADVANCED 2020EXERCISE - SECTION-3
6 Videos
Similar Questions
Match the anionic species given in column I that are present in the ore(s) given in column II.

View SolutionThe number of hydroxyl group(s) in Q is
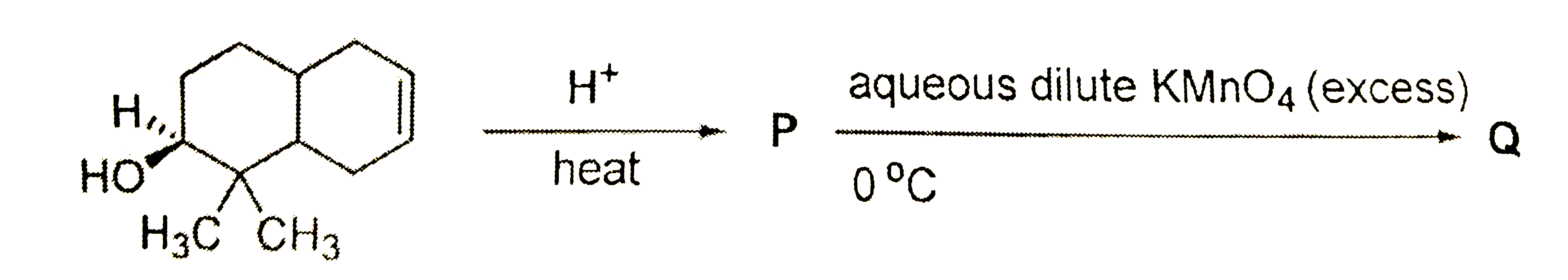
View SolutionThe total number of stereoisomers that can exist for M is

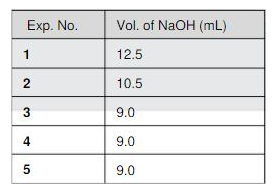
View Solution5.00 mL of 0.10 M oxalic acid solution taken in a conical flask is titrated against NaOH from a burette using phenolphthalein indicator. The volume of NaOH required for the appearance of permanent faint pink color is tabulated below for five experiments. What is the concentration, in molarity, of the NaOH solution?
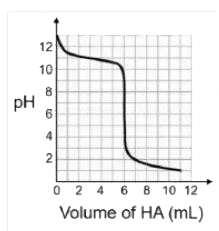
View SolutionA solution of 0 .1 weak base (B )is titrated with 0.1 M of a strong acid ( HA) . The variation of pH of the solution will be the volume of HA added is shown in the figure below. What is the pKb of the base ? The neutralization reaction is given by B+HA→HA→BH++A−
View SolutionN_(2)(g)+3H_(2)(g) hArr 2NH_(3)(g), DeltaH^(ɵ)=-22.4 kJ The pressure inside the chamber is 100 atm and temperature at 300 K The preparation of ammonia by Haber's process is an exothermic reaction. If the preparation follows the following temperature-pressure relationship for its % yield. Then for temperature T_(1), T_(2) and T_(3) the correct option is:
View SolutionConsider the equilibrium N_(2)(g) + 3H_(2)(g)hArr2NH_(3)(g) , Delta H = - 93.6 kJ . The maximum yield of ammonia obtained by
View SolutionThe % yield of ammonia as a function of time in the reaction, N_(2)(g)+3H_(2)(g)hArr2NH_(3)(g)'DeltaHlt0 at (p,T_(1)) is given below If this reaction is conducted at (p,T_(2)) , with T_(2)gtT_(1) the % yield by of ammonia as a function of time is represented by
View SolutionThe prepation of SO3(g) by reaction SO2(g)+12O2(g)⇔SO3(g) is an exothermic reaction .If the preparation follows the following temperature -pressure relationship for % yield , then for temperatures T1,T2andT3 the correct option is:
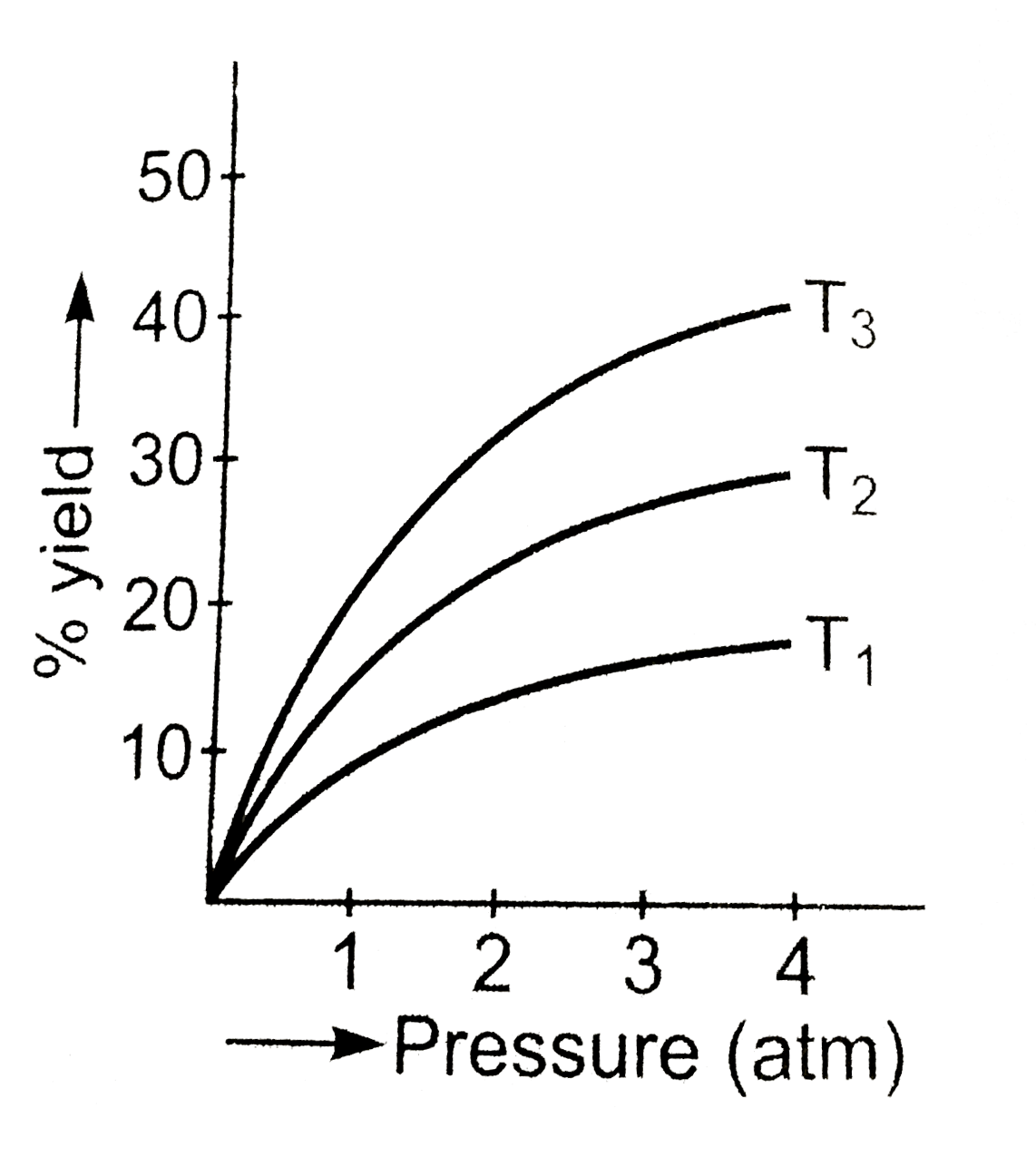
View SolutionIn reaction N2(g)+3H2(g)⇔2NH3(g),ΔH=−93.6kJ/mole, the yield of ammonia does not increase when
View SolutionKp is how many times equal to Kc for the given reaction ? N2(g)+3H2(g)⇔2NH3(g)
View SolutionThe % yield of ammonia as a function of time in the reaction N2(g)+3H2(g)⇔2NH3(g),(g)ΔH<0 at (P,T1) is given below
View SolutionIn reaction N2(g)+3H2(g)⇔2NH3(g),ΔH=−93.6kJ/mole, the yield of ammonia does not increase when
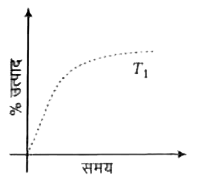
View Solutionअभिक्रिया, N2(g)+3H2(g)↔2NH3(g), ΔH<0 में अमोनिया के प्रतिशतता उत्पाद (% yield) की समय (time) पर निर्भरता (p,T1) पर नीचे दर्शाई गई है
यदि यह अभिक्रिया (p,T2) पर की जाए, जहाँ T2>T1, अमोनिया के % उत्पाद की समय पर निर्भरता प्रदर्शित करता है
View SolutionThe % yield of Ammonia as a function of time in the reaction N2(g)+3H2(g)⇔2NH3(g),ΔH<O at (P,T) is given below:
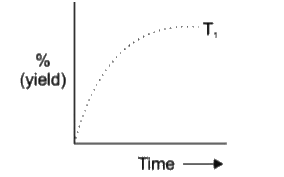
If this reaction is conducted at T2>T1, then plot the % yield of NH3 as a function of time on same graph
View Solutionअभिक्रिया N2(g)+3H2(g)<⇒2NH3(g) में ΔH=−93.6kJ है, अमोनिया की लब्धि (yield) नहीं बढ़ेगी, यदि
View Solution

