Figure shows an isochore, an isotherm, an adiabatic and two isobars of two gases on a work done versus heat supplied curve. The initial states of both gases are the same and the scales for the two axes are same.

Straight line 1 corresponds to an isochoric process.
Straight line 2 corresponds to an isobaric process for diatomic gas.
Straight line 4 corresponds to an isothermal process.
Straight line 5 corresponds to an isothermal process.
The correct Answer is:D
d. From the first law of thermodynamics
Q=W+ΔU
For an isobaric process
W=RΔT,Q=CPΔT=(f+22)RΔT
WQ=2f+2
where f is the number of degrees of freedom For an isochoric process.
W=0,WQ=0
The straight line 1 corresponds toan isochoric process.
For monatoic gas f=3 and for diatomic gas f=5.
WQ=25 (for monatomic gas)
=27 (for diatomic gas)
Straight line 2 corresponds to isobaric process for diatomic gas straight line 3 corresponds to isobaric process for monatomic gas.
For isotherm W=Q,tanθ4=1 straight line 4 only if ΔT=0 i.e., ΔU=0, For an adiabatic process Q=0, the straight line 5 corresponds to it.
Topper's Solved these Questions
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Multiple Corrects
Explore 28 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Assertion-Reasoning
Explore 6 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Subjective
Explore 22 Videos
- KINETIC THEORY OF GASESBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASESExercise:Compression
Explore 2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBook:CENGAGE PHYSICS ENGLISHChapter:LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONExercise:Single correct anwer type
Explore 14 Videos
Similar Questions
Under the same conditions, two gases have the same number of molecules. They must
View SolutionA certain mass of a gas is compressed first adiabatically, and then isothermally. In both cases, the initial state of the gas is the same. Is the work done W1 in the first case greater than the work done W2 in the second case ? Explain.
View SolutionThe straight lines in the figure depict the variations in temperature ΔT as a function of the amount of heat supplied Q is different process involving the change of state of a monoatomic and a diatomic ideal gas . The initial states, (P,V,T) of the two gases are the same . Match the processes as described, with the straight lines in the graph as numbered.
_E01_155_Q01.png)
Column-IColumn-II(A)Isobaric process of monoatomic gas.(p)1(B)Isobaric process of diatomic gas(q)2(C)Isochoric process of monoatomic gas(r)Minimum at section B(D)Isochoric process of diatomic gas(s)x-axis (i.e.'Q' axis)
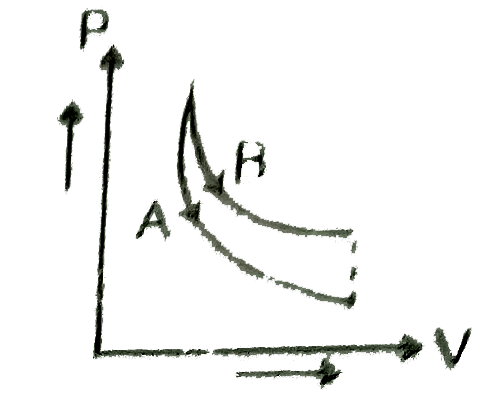
View SolutionIn figure, A and B are two adiabatic curves for two different gases. Then A and B corresponds to:
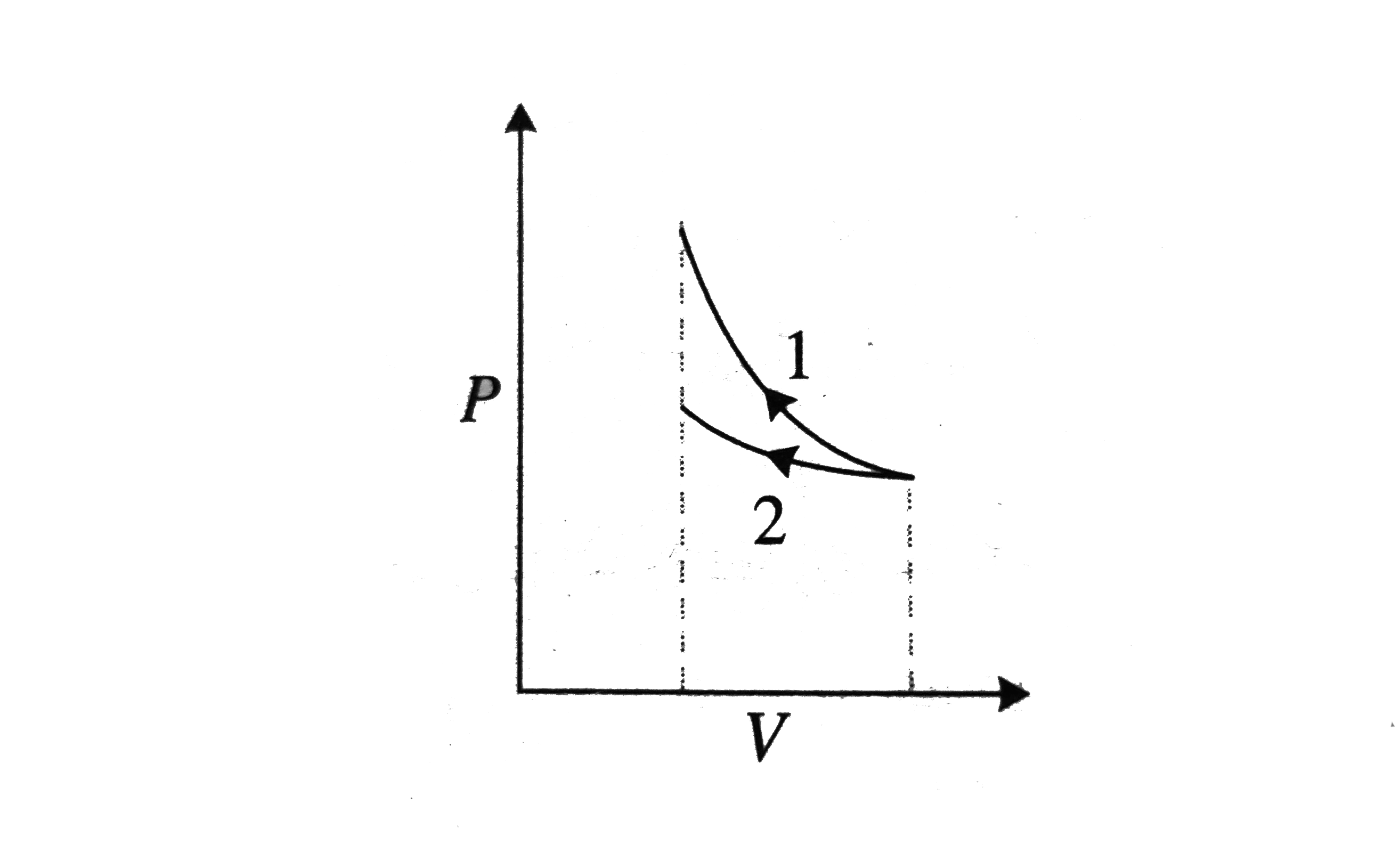
View SolutionConsider two processes on a system as shown in figure.
The volumes in the initial states are the same in the two processes and the volume in the final states are also the same.Let ΔW1 and ΔW2 be the work done by the system in the processes A and B respectively.
View SolutionConsider two processes on a system as shown in figure.
The volumes in the initial states are the same in the two processes and the volume in the final states are also the same.Let ΔW1 and ΔW2 be the work done by the system in the processes A and B respectively.

View Solutionconsider two processes on a system as shown in fig
the volumes in the initial stage are the same in the two processes and the volume in the final stage are also the same.Let ΔW1andΔW2 be the work done by the system in the processes A and B respectively.

View SolutionIn the arrangement shown in the fig the cylinder is insulating one. Both sides same diatomic gas is trapped by two insulting massless pistos with the help of an ideal spring. The natural length of the spring is equal to the length of the cylinder. initial state of the gases are as shown in the figure.

What is the value of energy stored in the spring ?
View SolutionA mixture of two gases A and B in the mole ratio 2:3 is kept in a 2 litre vessel. A second 3 litre vessel has the same two gases in the mole ratio 3:5. Both gas mixtures have the same temperature and same pressure. They are allowed to intermix and the final temperature and pressure are the same as the initial values, the final volume beingh 5 litres. Given theat the molar masses are MA and MB . what is the mean molar mass of the final mixture :
View SolutionStatement-1: Two gases at same pressure, volume & temperature are compressed to same volume. The first isothermally and second adiabatically, greater work is done on the gas for an adiabatic process.
Statement-2: Final temperature for gas going under isothermal process is more than gas going under adiabatic process.
View Solution
