Refer to figure. Let ΔU1 and ΔU2 be the changes in internal energy in the system in process A+B and ΔW be the net work done by the system in the process A+B,
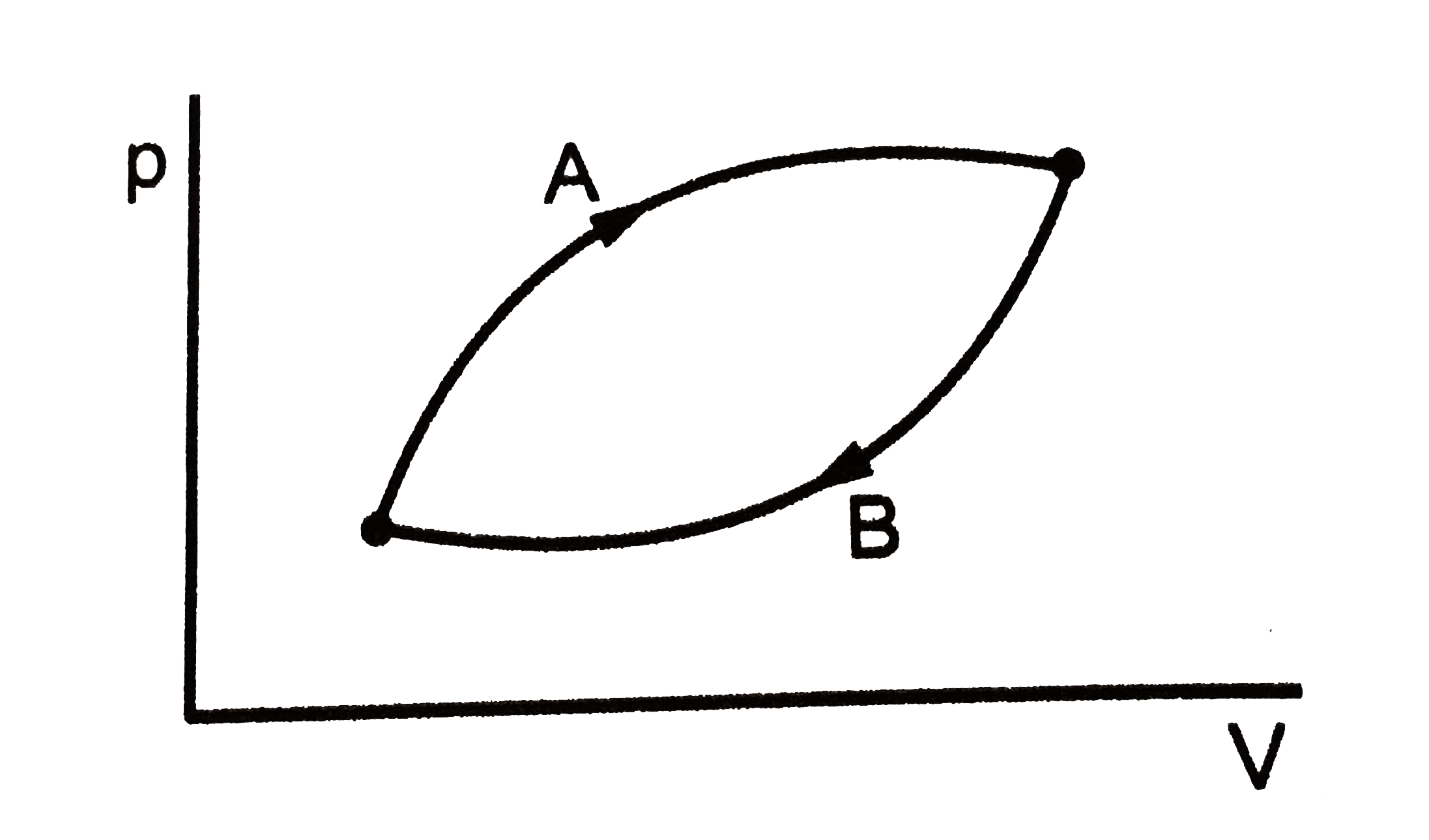
ΔU1+ΔU2=0
ΔU1−ΔU2=0
ΔQ−ΔW=0
ΔQ+ΔW=0
The correct Answer is:A, C
For a cycle process, ΔU=0
i.e., ΔU=ΔU1+ΔU2=0
From relation ΔQ=ΔU+ΔW
As ΔU=0
Hence, ΔQ=ΔW
or ΔQ−ΔW=0
Topper's Solved these Questions
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Assertion-Reasoning
Explore 6 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Comprehension
Explore 56 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Single Correct
Explore 140 Videos
- KINETIC THEORY OF GASESBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASESExercise:Compression
Explore 2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBook:CENGAGE PHYSICS ENGLISHChapter:LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONExercise:Single correct anwer type
Explore 14 Videos
Similar Questions
Statement I: A quasi -static process is so called bacause it is a sudden and large change of the system.
Statement II: An adiabatic process is not quasi- static because it is a sudden and large change of the system.
View SolutionA fixed mass of gas is taken through a process A→B→C→A. Here A→B is isobaric, B→C is adiabatic and C→Ais isothermal. Find pressure at C
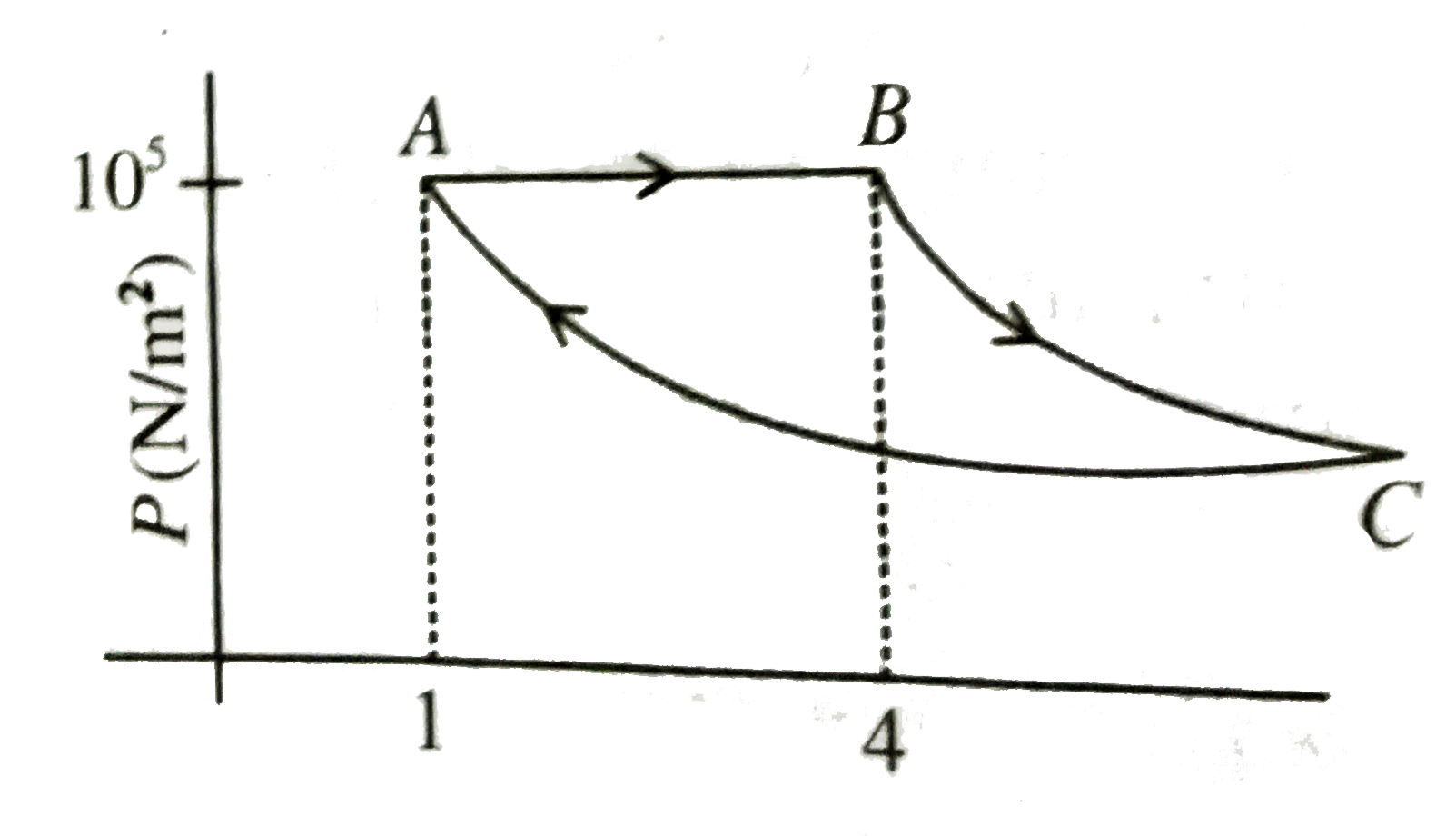
.
View SolutionThe temperature of a gas is raised while its volume remains constant, the pressure exerted by the gas on the walls of the container increases because its molecules
View SolutionOne mole of an ideal monatomic gas undergoes a linear process from A to B , in which is pressure P and its volume V change as shown in figure .
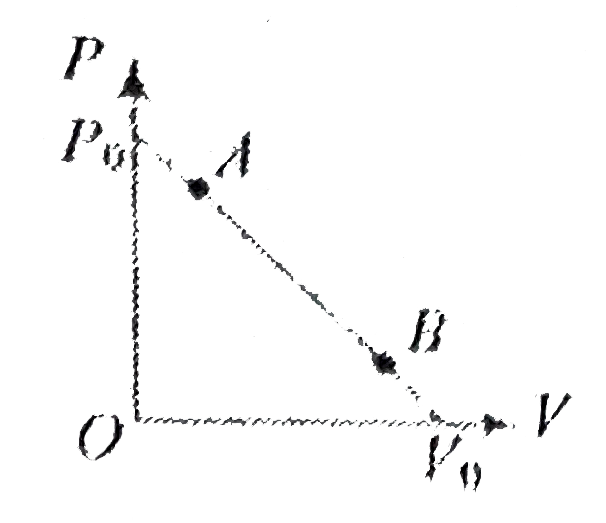
The absolute temperature T versus volume V for the given process is
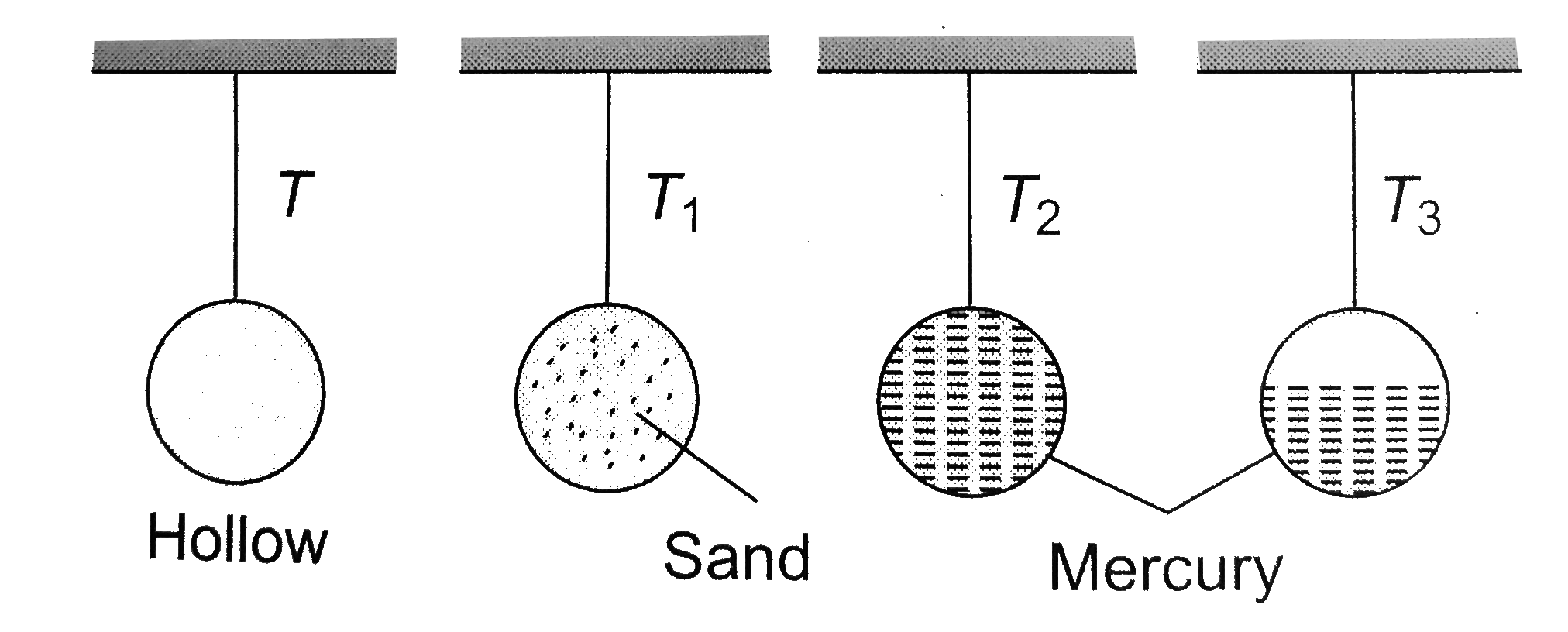
View SolutionThe period of a simple pendulum whose bob is hollow metallic sphere is T. The period is T1 when the bob is filled with sand, T2 where it is filled with mercury and T3 when it is half filled with mercury Which of the following is true?
View SolutionRefer to let DeltaU_(1) and DeltaU_(2) be the changes in internal energy of the system in the processes A and B. then
View SolutionRefer to let DeltaU_(1) and DeltaU_(2) be the changes in internal energy in the system in process A+B and DeltaW be the net work done by the system in the process A+B,
View SolutionAn ideal gas goes from State A to state B via three different process as indicate in the P-V diagram. If Q_(2), Q_(3) indicates the heat absorbed by the gas along the three processes and DeltaU_(1), DeltaU_(2), DeltaU_(3) indicates the change in internal energy along the three processes respectively, then
View SolutionIn given figure, let DeltaU_(1) and DeltaU_(2) be change in internal energy in process ABC and CDA respectively. DeltaQ and W be the net heta given and net work done by the system in the process ABC +CDA , then
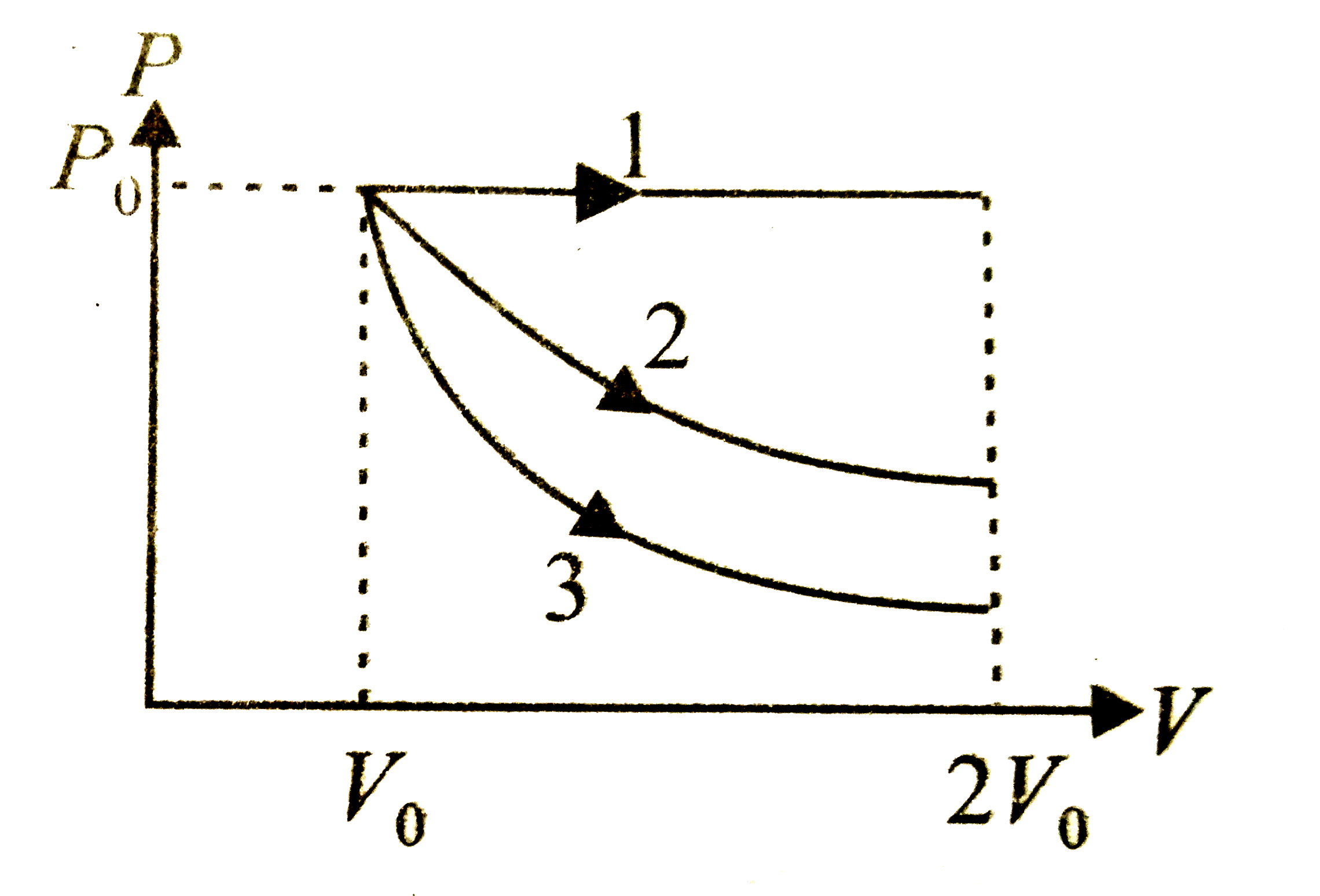
View SolutionA gas is expanded form volume V_(0) to 2V_(0) under three different processes as shown in the figure . Process 1 is isobaric process process 2 is isothermal and and process 3 is adiabatic . Let DeltaU_(1),DeltaU_(2) and DeltaU_(3) be the change in internal energy of the gs in these three processes then
View SolutionRefer to figure. Let ΔU1 and ΔU2 be the changes in internal energy of the system in the processes A and B. then
View SolutionRefer to figure. Let ΔU1 and ΔU2 be the changes in internal energy in the system in process A+B and ΔW be the net work done by the system in the process A+B,

View SolutionA body process 1 changes from stage A to stage B by process 2, as pictured. If the internal energy changes in process 1 DeltaU_(1) And internal energy changes in process 2 DeltaU_(2) Is then
View SolutionA gas is expanded form volume V0→2V0 under three different processes as shown in the figure . Process 1 is isobaric process process 2 is isothermal and and process 3 is adiabatic .
Let ΔU1,ΔU2andΔU3 be the change in internal energy of the gas in these three processes then
View Solutionएक गैस चित्र में दर्शाए अनुसार, तीन विभिन्न प्रक्रमों में V0 से 2V0 आयतन तक प्रसारित की जाती है। प्रक्रम 1 समदाबी प्रक्रम है, प्रक्रम 2 समतापी तथा प्रक्रम 3 रुद्धोष्म है।
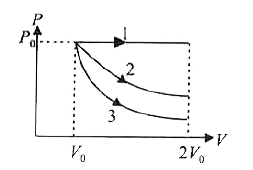
माना ΔU1,ΔU2 एवं ΔU3 इन तीनों प्रक्रमों में गैस की आन्तरिक ऊर्जा में परिवर्तन हैं, तो
View SolutionReferring to figure of previous question let DeltaU_(1) and DeltaU_(2) be the changes in internal energy of the system in the processes A and B. Then
View Solution
