Hybridisation in [Co(NH3)6]3+ is
A
sp3
B
sp3d
C
d2sp3
D
sp3d3
Video Solution
Text Solution
The correct Answer is:C
Valence shell electronic configuration of Co2+
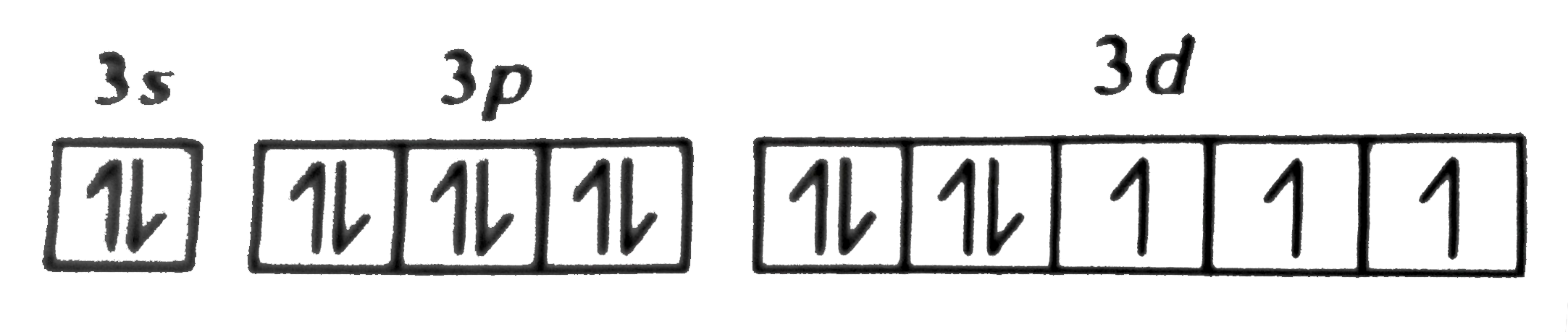
Formation of [Co(NH3)2+60 complex
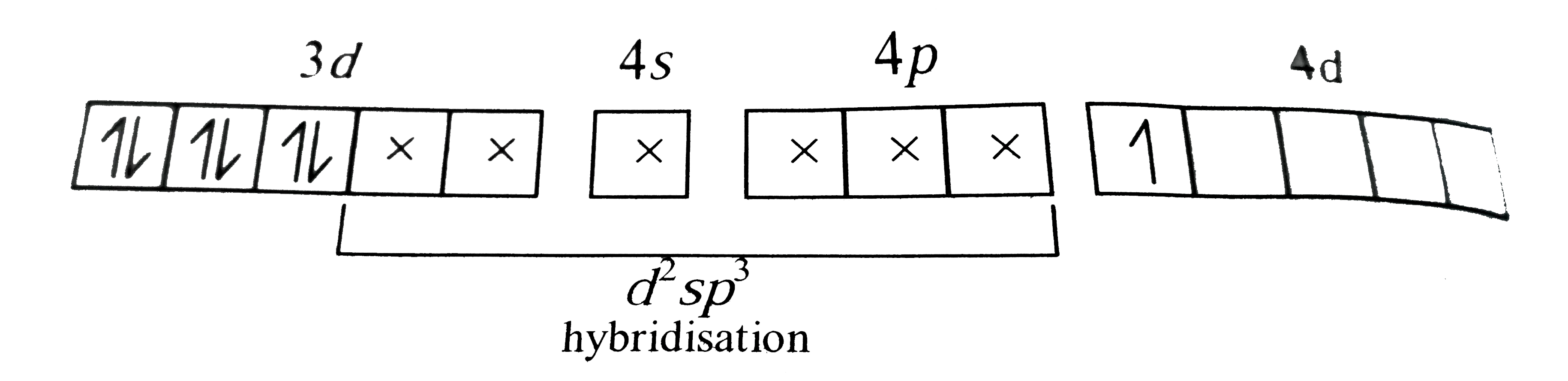
|
Topper's Solved these Questions
- COORDINATION CHEMISTRYBook:ERRORLESS Chapter:COORDINATION CHEMISTRYExercise:ORDINARY THINKING Objective Questions (COMPLEXES AND COMPLEX STABILITY)
Explore 17 Videos
- COORDINATION CHEMISTRYBook:ERRORLESS Chapter:COORDINATION CHEMISTRYExercise:ORDINARY THINKING Objective Questions (APPLICATION OF ORGANOMETALLICS)
Explore 13 Videos
- COORDINATION CHEMISTRYBook:ERRORLESS Chapter:COORDINATION CHEMISTRYExercise:ORDINARY THINKING Objective Questions (Isomerism and Magnetic properties)
Explore 86 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODIC PROPERTIES Book:ERRORLESS Chapter:CLASSIFICATION OF ELEMENTS AND PERIODIC PROPERTIES Exercise:JEE Section Matrix Match type questions
Explore 2 Videos
- D & P-BLOCK ELEMENTSBook:ERRORLESS Chapter:D & P-BLOCK ELEMENTSExercise:JEE Section (Numeric answer type questions)
Explore 2 Videos
Similar Questions
Knowledge Check
Two statements 'A' and 'R' are given below Select your answers to these items using the codes given below:
Assertio (A) : Among the cobalt (III) complexes [Co(NH3)6]3+ is a diamagnetic but [CoF6]3− is paramagnetic.
Reason (R) : Hybridisation of [Co(NH3)6]3+ is sp3d2 where as hybridisation of Co in [CoF6]3− is d2sp3.ABoth A and R are correct and R is the correct explanation of ABBoth A and R are correct and R is not a correct explanation of ACA is true but R is falseDA is false but R is trueThe hybridization in [CO(NH3)6]3+ is :
Adsp2Bsp3d2Csp3Dd2sp3The hybridisation of Co in [Co(H2O)6]3+ is:
Ad2sp3Bdsp2Csp3Dsp3d2
The crystal field stabilisation energy of [Co(NH3)6]Cl3 is:
View SolutionThe crystal field stabilisation energy of [Co(NH3)6]Cl3 is:
View SolutionThe type of hybridisation present in [Cu(NH3)4]+2 ion is
View SolutionThe coordination compounds, [Co(NH3)6]3+,[Cr(CN)6]3− and [Cr(NH3)6]3+[Co(CN)6]3− are examples of
View SolutionStatement-1: Complex ion [Co(NH3)6]2+ si readily oxidized to [Co(NH3)6]3+.
Statement-2: Unpaired electron in complex ion [Co(NH3)6]2+ is present in 4d orbital.
View Solution
