For any reaction, greater the value of equilibrium constant greater is the extent of reaction.
Video Solution
Text Solution
1
|
Similar Questions
Knowledge Check
The value of equilibrium constant for a feasible cell reaction is :
A<1B=1C>1DzeroIf the equilibrium constant for a reaction is 4⋅0 , what will be the equilibrium constant for the reverse reaction.
A1B4C0.25D25Equilibrium constant of a reaction is elated to :
AStandard free energy change ΔG∘BFree energy change ΔGCEntropy changeDNone
The equilibrium constant for the following reaction is
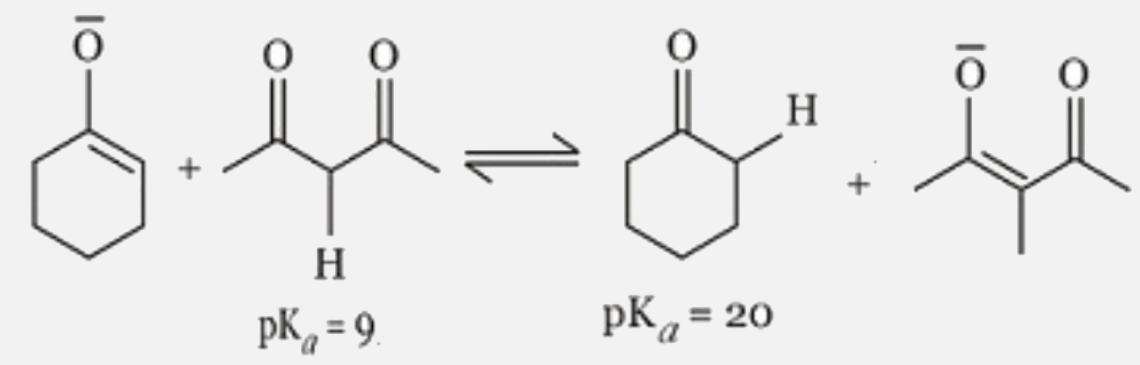
View SolutionThe reaction having higher value of equilibrium constant is faster than the reaction having lower value of equilibrium constant.
View SolutionIf the concentration quotient of a reaction in greater than its equilibrium constant, then the reaction will proceed in the ______ direction.
View SolutionEquilibrium constant of a reaction is related to
View SolutionAssertion : The equilibrium constant for the reverse reaction is equal to the inverse of the equilibrium constant for the forward reaction .
Reason : The value of equilibrium constant is independent of initial concentrations of the reactants and products.
View Solution
