Which of the following represents the chemical equation involved in the preparation of H2O2 from barium peroxide ?
BaO2.8H2O+H2SO4→BaSO4+H2O2+8H2O
CH3CHOHCH3+O2→CH3COCH3+H2O2
BaO2+CO2+3H2O→BaCO3+H2O2
Ba3(PO4)2+3H2SO4→3BaSO4+2H3PO4
The correct Answer is:A
BaO2.8H2O(s)+H2SO4(aq)→BaSO4(s)+H2O2(aq)+8H2O(l)
Topper's Solved these Questions
- HYDROGEN Book:NCERT FINGERTIPSChapter:HYDROGEN Exercise:Heavy Water
Explore 8 Videos
- HYDROGEN Book:NCERT FINGERTIPSChapter:HYDROGEN Exercise:Higher Order Thinking Skills
Explore 9 Videos
- HYDROGEN Book:NCERT FINGERTIPSChapter:HYDROGEN Exercise:MCQs
Explore 1 Video
- HYDROCARBONS Book:NCERT FINGERTIPSChapter:HYDROCARBONS Exercise:Assertion And Reason
Explore 15 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES Book:NCERT FINGERTIPSChapter:ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES Exercise:Assertion And Reason
Explore 12 Videos
Similar Questions
What is heavy water ?
View SolutionThe varous types of hydrides and examples of each type are given below :
Hydride typeCompound(A)Electron deficient(i)LiH(B)Saline(ii)CH4(C)Electron-precise(iii)NH3(D)Interstitial(iv)B2H6(E)Electron rich(v)CrH
Choose the correct matching from the codes given below :
View SolutionChoose the correct statement about the given figures.
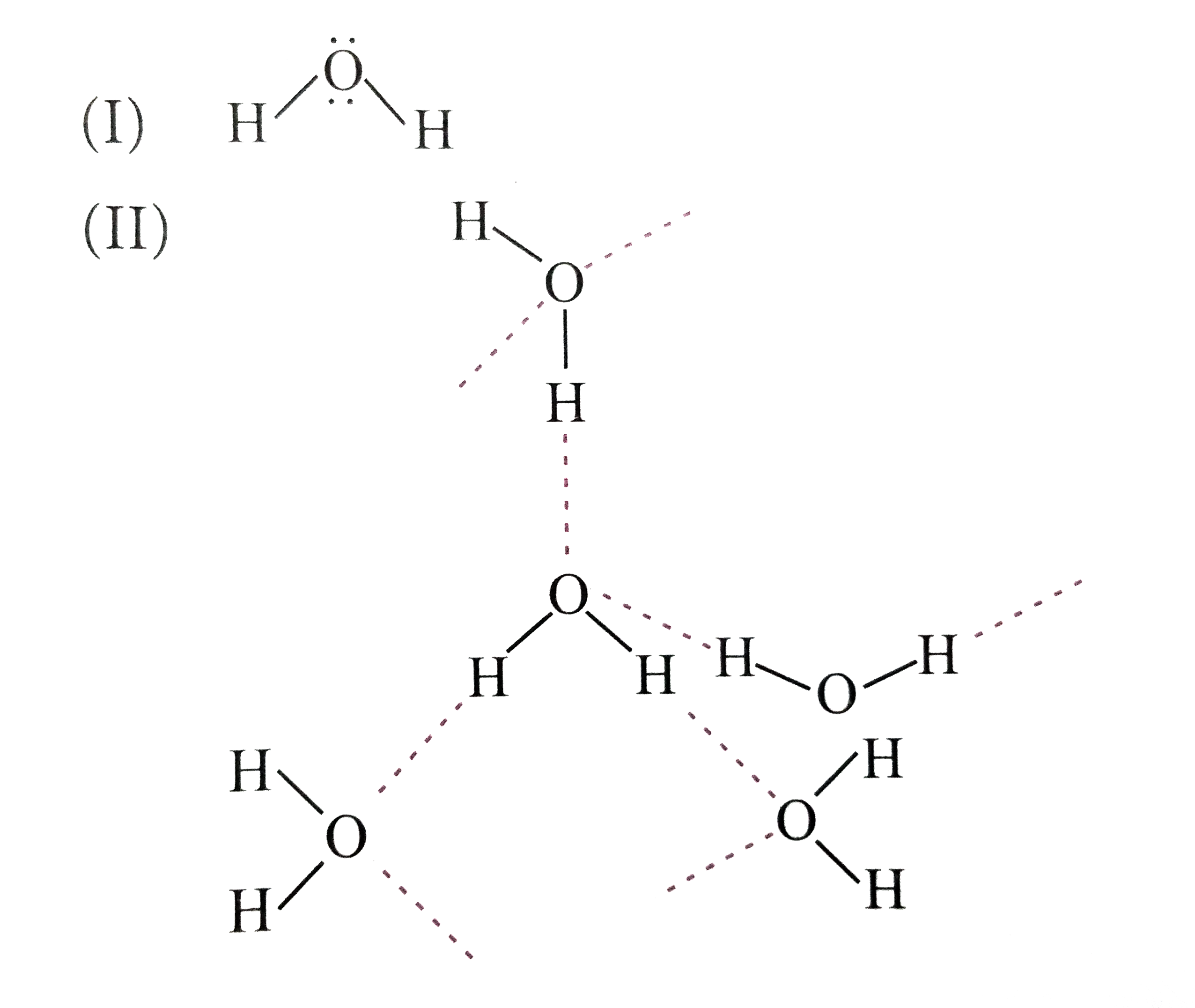
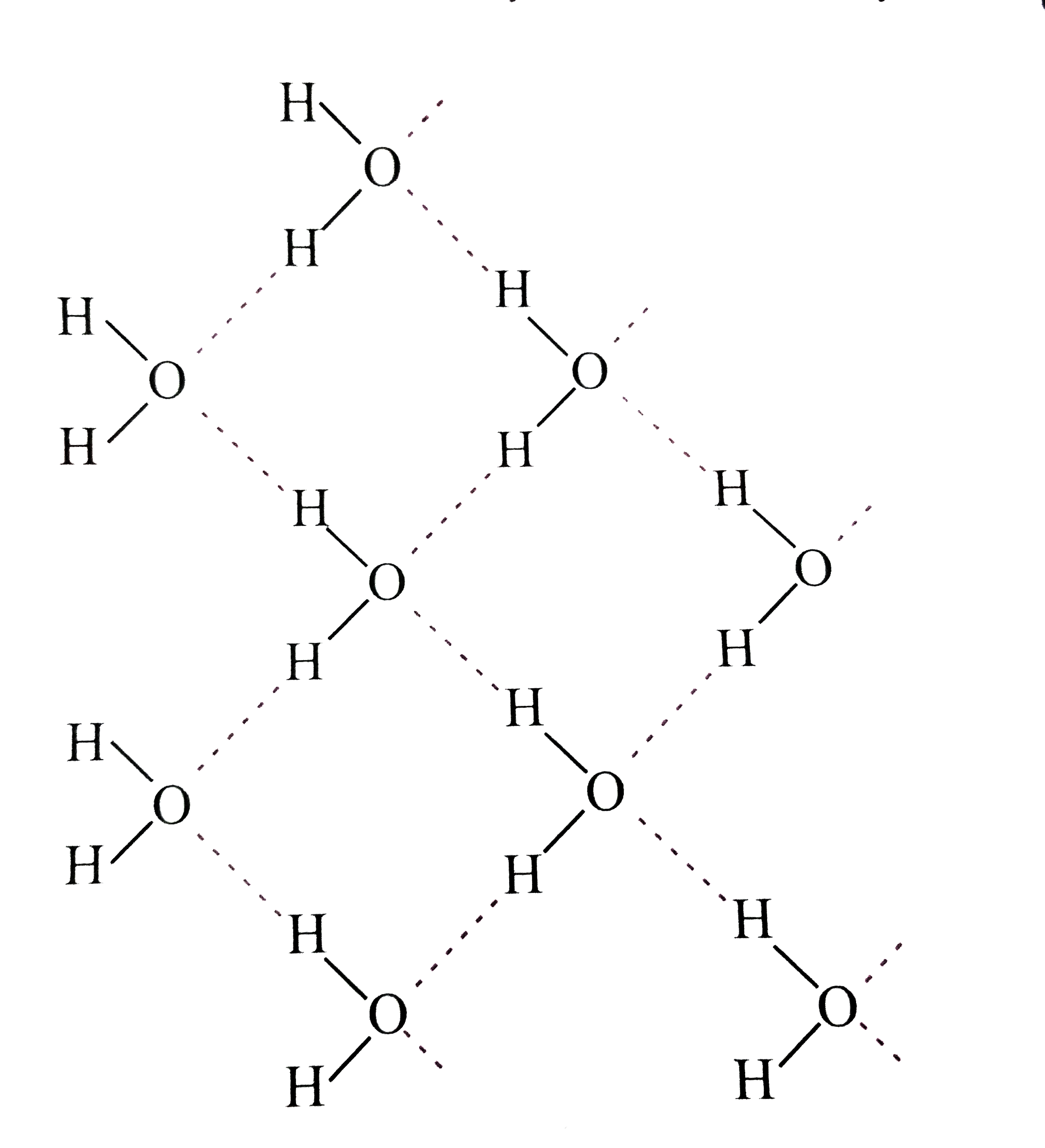
View SolutionAssertion: 2,2-Dimethylbutane does not have any tertiary carbon atom.
Reason: Tertiary carbon atom is attached to three carbon atoms.
View SolutionAssertion: Hybridisation influences the bond length and bond enthalpy in organic compound.
Reason: More the s character of hybrid orbital, shorter and stornger will be the bond.
View SolutionExplain the following.
a. Hydrated barium peroxide is used in the preparation of H2O2 instead of anhydrous barium peroxide.
b. Phosphoric acid is preferred to sulphuric acid in the preparation of H2O2 from barium peroxide.
c. Hydrogen is not prepared by action of concentrated sulphuric acid on zinc.
d. A solution of ferric chloride is unaffected when hydrogen is bubbled through it, but gets reduced when zinc is added to the same acidified solution.
View SolutionH_(2)O_(2)rarr 2H^(+)+O_(2)+2e^(-),E^(@)=-0.68V . This equation represents which of the following behaviour of H_(2)O_(2)
View SolutionExplain why phosphotric acid is preferred to sulphuric acid in preparation of H_(2)O_(2) from hydrated barium perioxide.
View SolutionWhy is hydrated barium peroxide used in the preparation of hydrogen peroxide instead of anhydrous barium peroxide ?
View Solutionरासायनिक समीकरण देते हुए बेरियम पराक्साइड से H2O2 के बनाने की विधि लिखिए।
View SolutionDiscuss, with relevant chemical equations, various methods of preparing hydrogen peroxide. Which of these methods is useful to prepare D_(2)O_(2) ?
View SolutionWrite the equation involved in the preparation of hydrogen peroxide from 2 - ethylanthraquinol.
View SolutionExplain why in the laboratory preparation of H_(2)O_(2) : A thin paste of hydrated barium peroxide is used instead of anhydrous barium peroxide
View SolutionHow is barium peroxide prepared?
View SolutionHow is barium peroxide prepared?
View Solution

