For the reaction between A and B
A+B→ product
the following rate data were obtained .
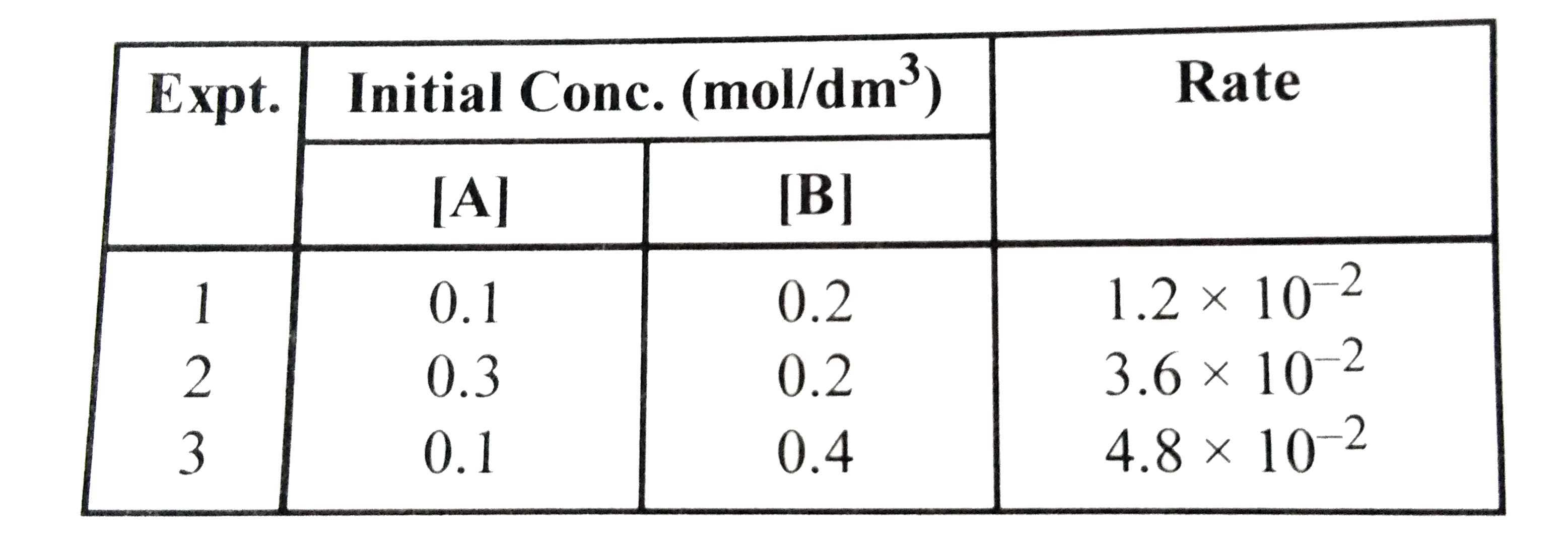
the reaction is
Zero order
first order
second order
third order
The correct Answer is:D
Rate =K[A]x[B]y
for expt , (1) 1.2×10−2=k[0.1]x[0.2]y
(2)3.6×10−2=k[0.3]x[0.2]y
(3)4.8×10−2=k[0.3]x[0.2]y
Dividing II by I,3=[3]x∴x=1
Dividing III by ,I ,4 =[2]y∴y=2
order of reaction x+y=1+2=3
Similar Questions
What will be the order of reaction of the type A→ Product for which the following data were obtained ?

View SolutionFor a reaction in which A and B form C, the following data were obtained form theee experiments:
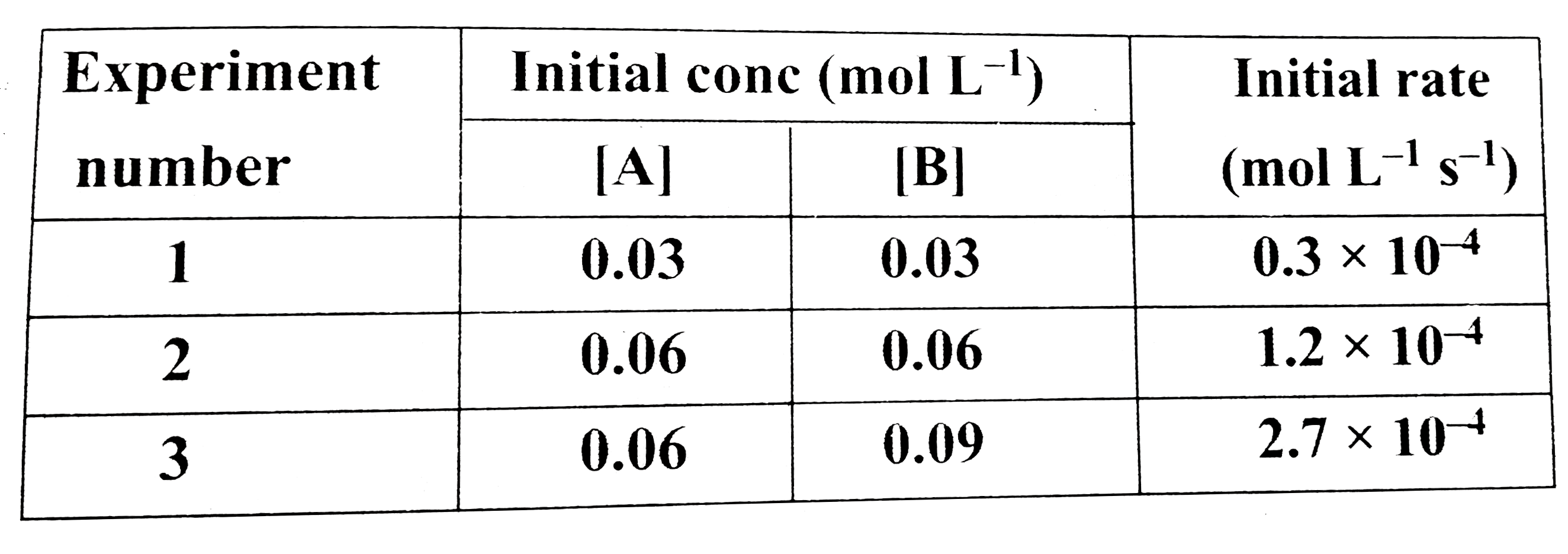
What is the rate equation of the equation and what is the value of rate constant ?
View Solution
Knowledge Check
For a creactionj, the following data were obtained:
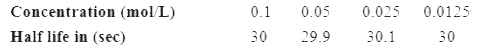
the order of reaction is:A2B1C0DfractionalProduct (B) of the following reaction is
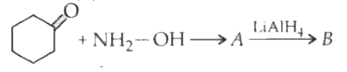 A
A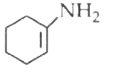 B
B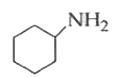 C
C D
D
Product (B) in the following reaction is
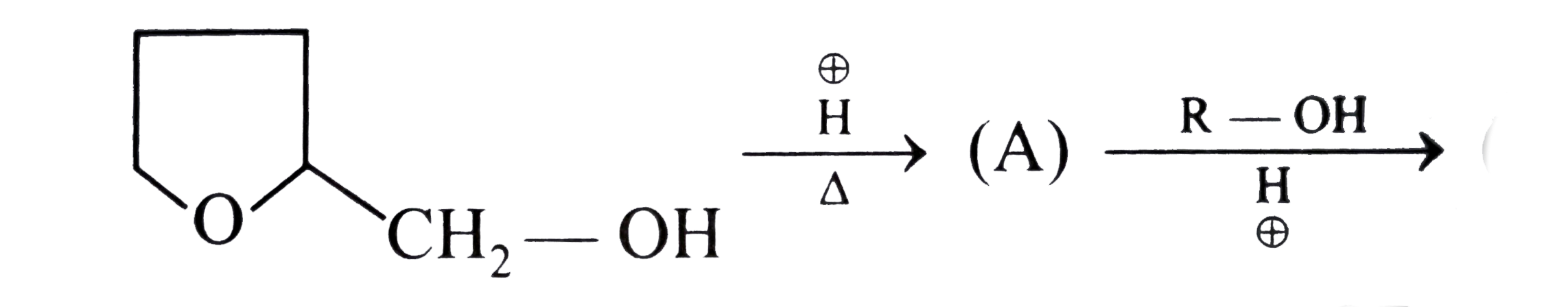 A
A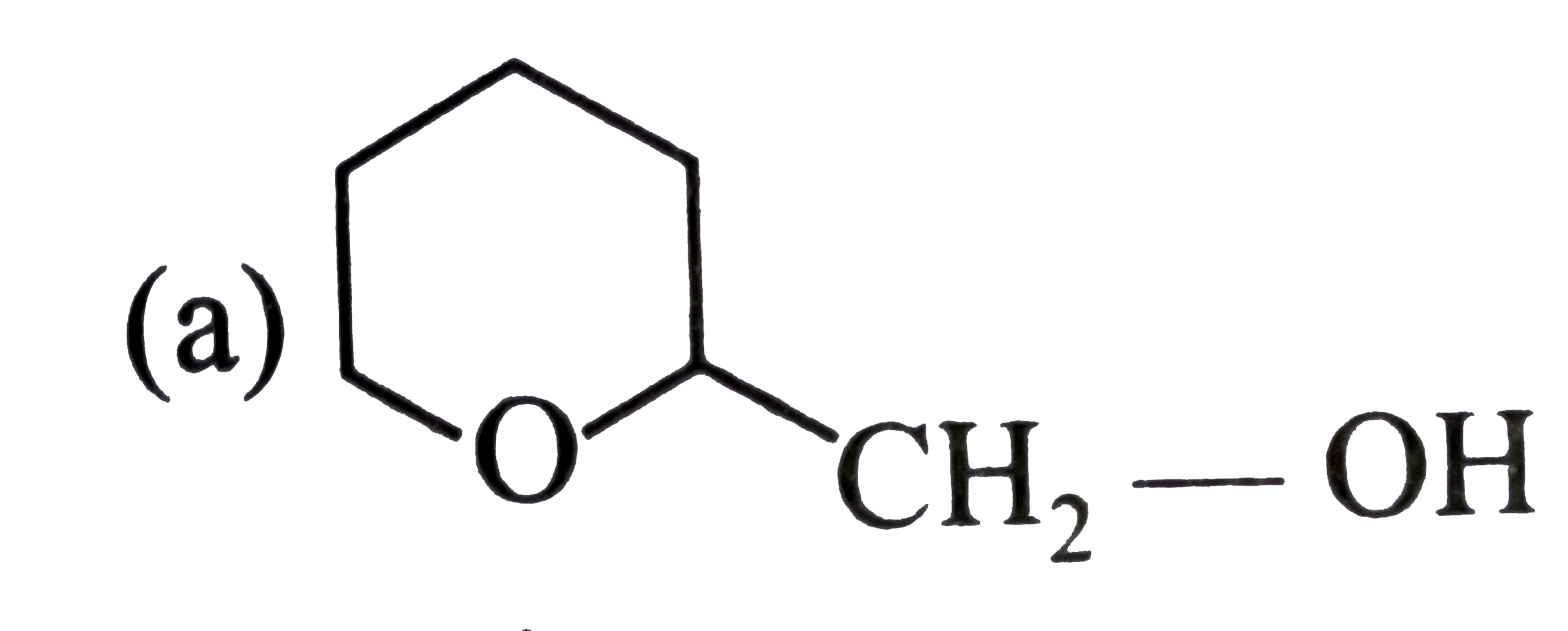 B
B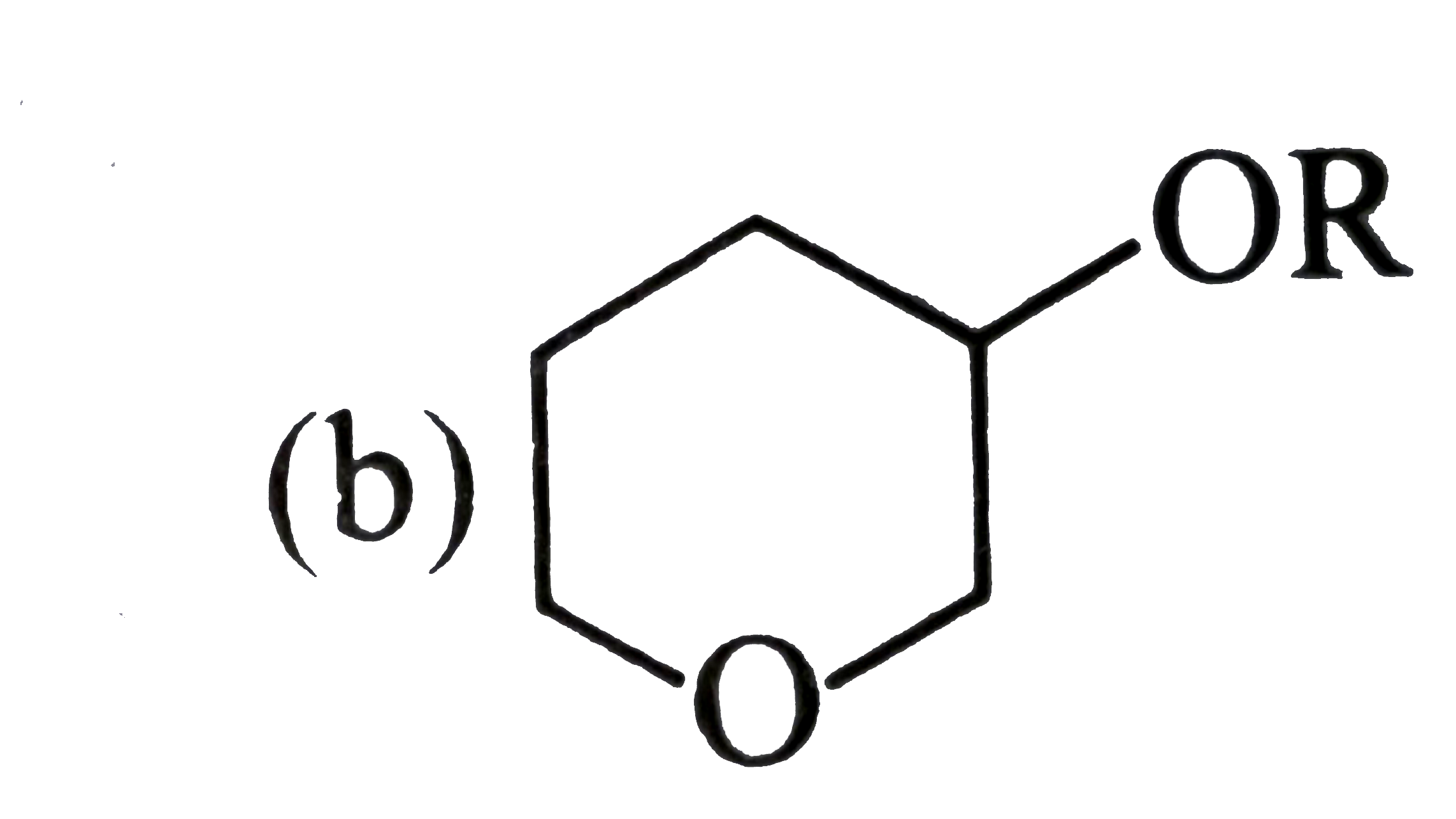 C
C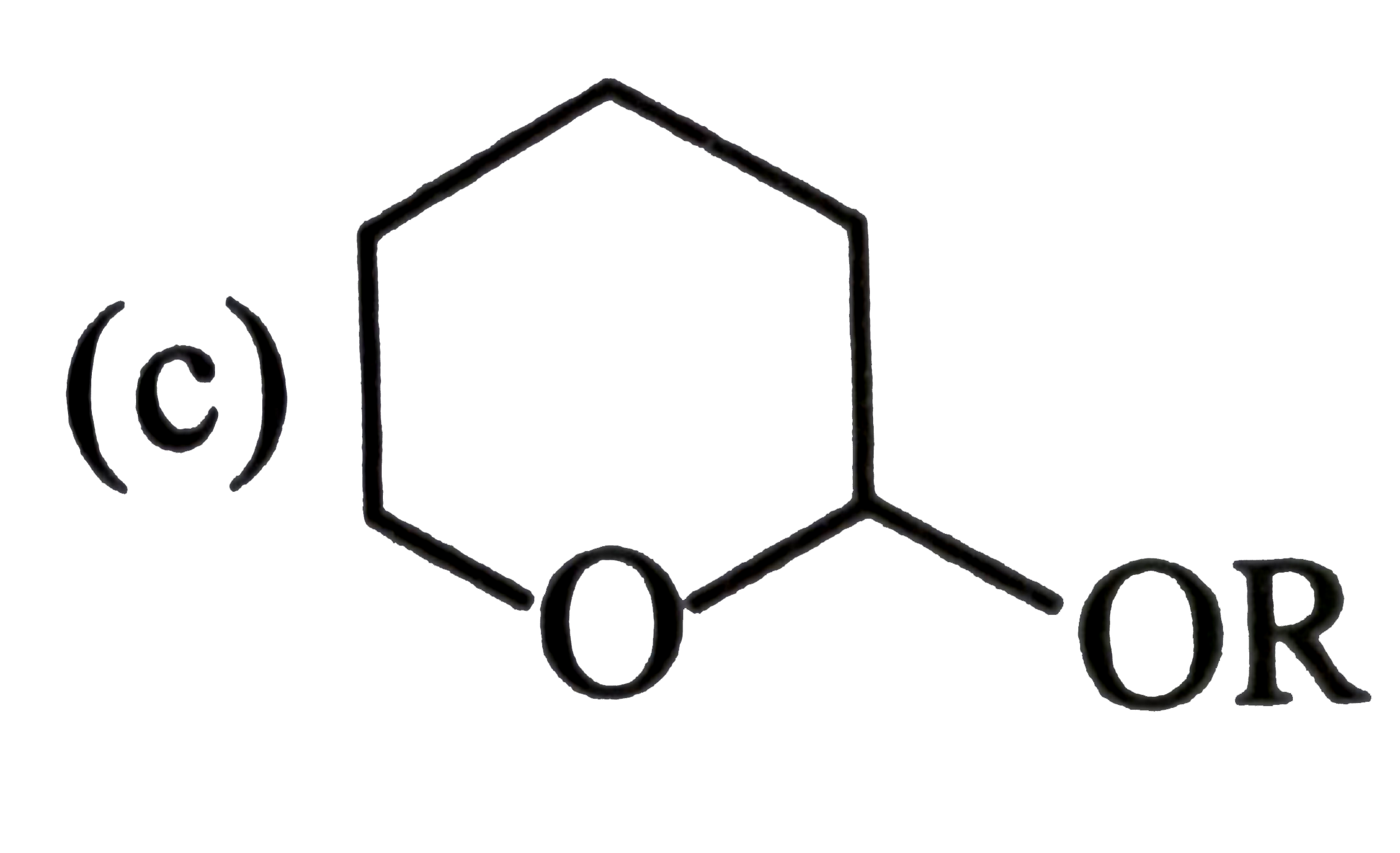 D
D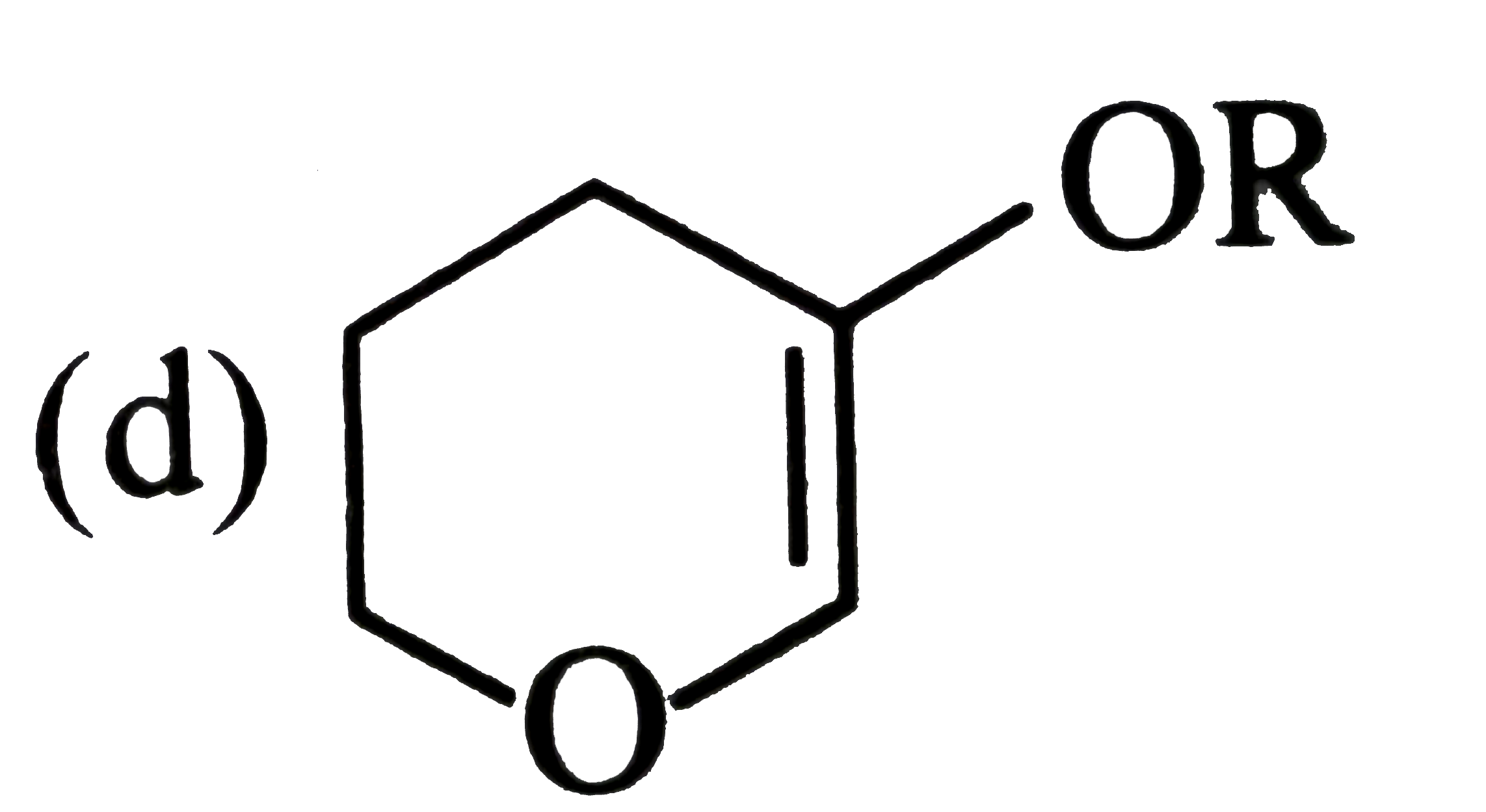
form the gaseous reaction
2A+B2→2AB, the following rate data were obtained at 300K.
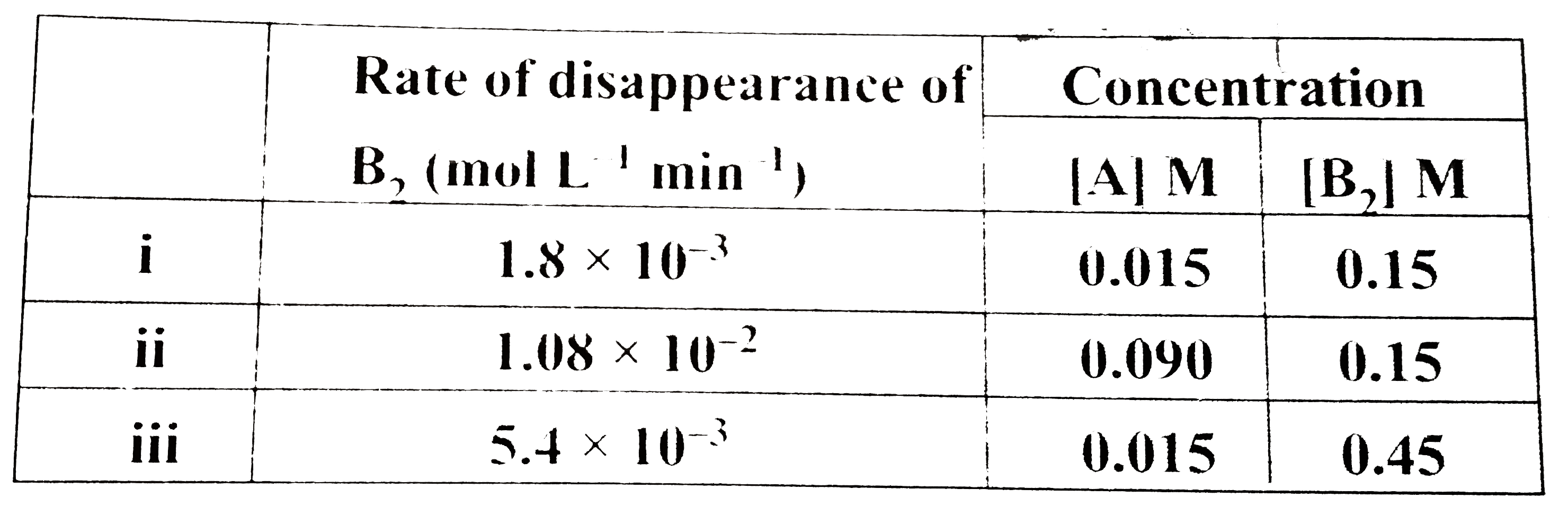
Calculate the rate constant for the reaction and the rate of formation of AB when [A] is 0.02 and [B2] is 0.04molL−1 at 300K.
View SolutionThe final product B obtained in the reaction is:
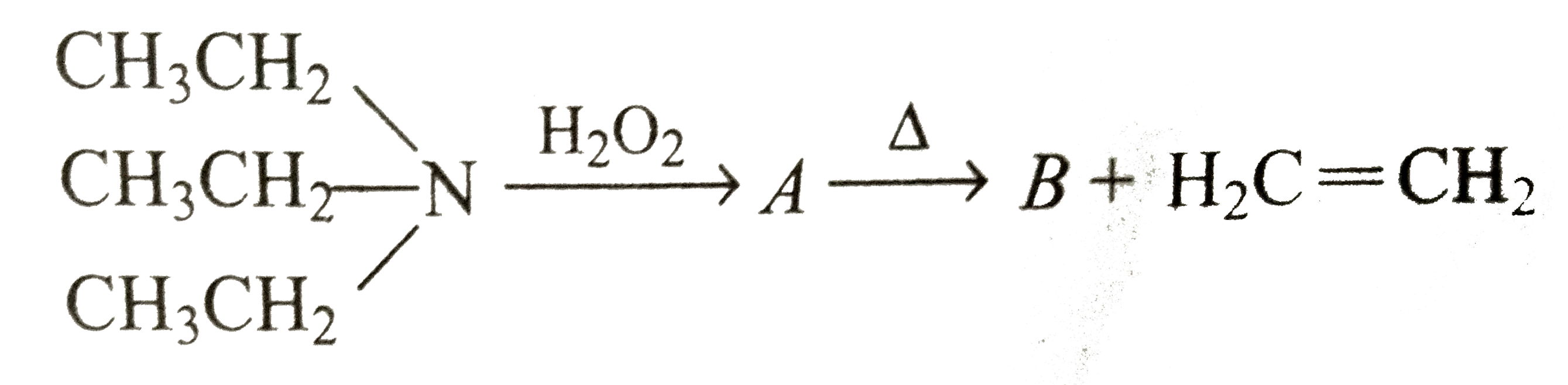
View SolutionFor a gaseous reaction, the following data were recorded :
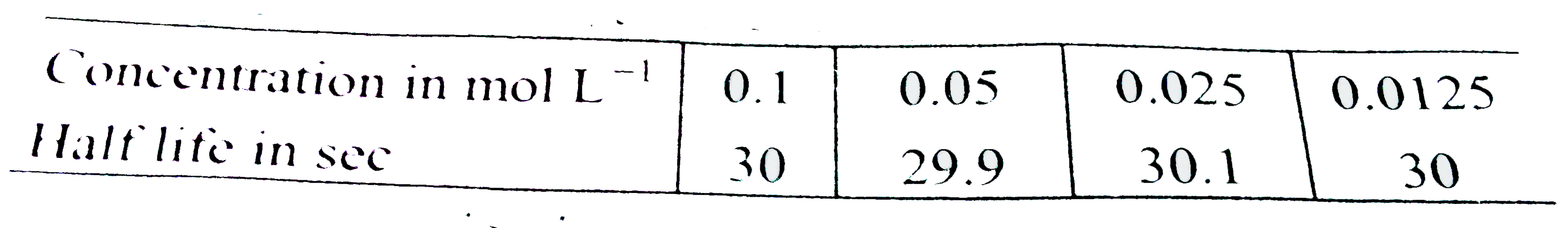
The order of reaction is :
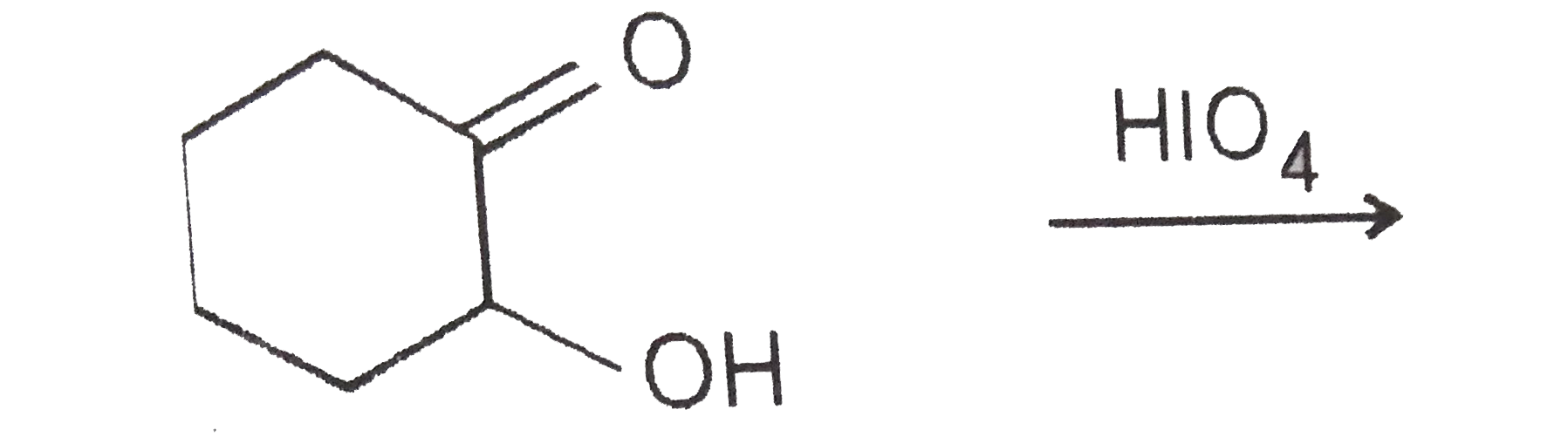
View SolutionThe product obtained in the following reaction is
View SolutionConsider the following reactions :
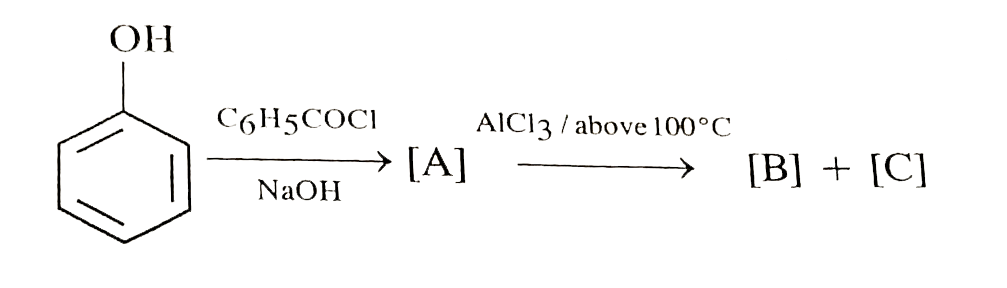
The major product [B] of the reaction is :
View Solution
