The reactivity of the compounds
(i) MeBr
(ii) PhCH2Br
(iii) MeCl
(iv) p−MeOC6H4 Br decreases as
(i)gt(ii)gt(iii)gt(iv)
(iv)gt(ii)gt(i)gt(iii)
(iv)gt(iii)gt(i)gt(ii)
(ii)gt(i)gt(iii)gt(iv)
The correct Answer is:D
Reactivity of halo compounds.
Similar Questions
Knowledge Check
What will be the correct order of the reactivity of the compounds (I) MeBr (II) PhCH2Br (III) MeCl towards nucleophilic substitution reactions?
A(I)>(II)>(III)B(III)>(I)>(II)C(II)>(I)>(III)D(I)>(III)>(IV)SN1 reactivity of the following halides will be in the order
> (i) (CH3)3Br (ii) (C6H5)2CHBr
> (iii) (C6H5)2C(CH3)Br (iv) (CH3)2CHBr
> (v) C2H5BrA(v) > (iv) > (i) > (ii) > (iii)B(ii) > (i) > (iii) > (v) > (iv)C(i) > (iii) > (v) > (ii) > (iv)D(iii) > (ii) > (i) > (iv) > (v)The correct order of reactivity of the compounds
(I) C6H5H2(Br), (II) C6H5CH(C6H5)Br, (III) C6H5CH(CH3)Br, (IV) C6H5C(CH3)(C6H5)Br in the increasing order of reactivity in SN2 reactions isA(I) lt (II) lt (III) lt (IV)B(IV) lt (III) lt (II) lt (I)C(I) lt (III) lt (II) lt (IV)D(IV) lt (II) lt (III) lt (I)
Explain the relative rates of RX with H2O+EtOH at 25∘C as given :
(i) MeBr(2140)
(ii) MeCH2Br(171)
(iii) Me2CHBr(4.99)
(iv) Me3CBr(1010)
(b) Why is EtOH added to water ?
View SolutionExplain the relative rates of RX with H2∅EtOH at 25∘C as given :
(i) MeBr(2140)
(ii) MeCH2Br(171)
(iii) Me2CHBr(4.99)
(iv) Me3CBr(1010)
(b) Why is EtOH added to water ?
View SolutionReactivity of HCHO with the following G.R in the decreasing order is :
(i) PhMgBr
(ii) PhCH2MgBr
(iii)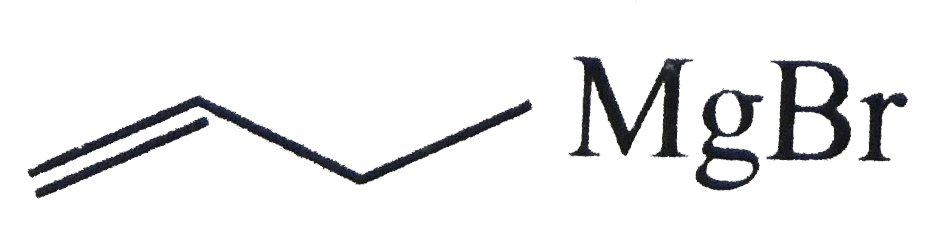
(iv)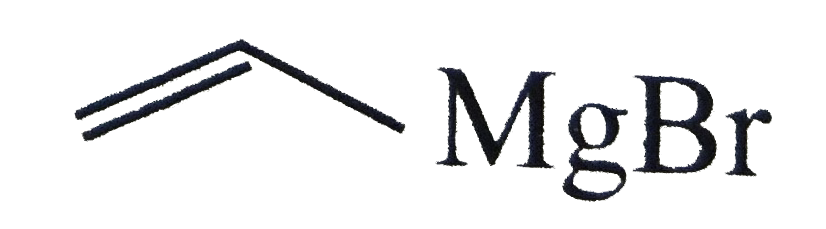 .
.
View SolutionThe SN1 reactivity of the following halides will be in the order:
(i) (CH3)3C−Br
(ii) (C6H5)CHBr
(iii) (C6H5)2C(CH3)Br
(iv) (CH3)2CHBr
(v) C2H5Br
View SolutionReactivity of PhMgBr with the following in the decreasing order is :
(i) PhCHO
(ii)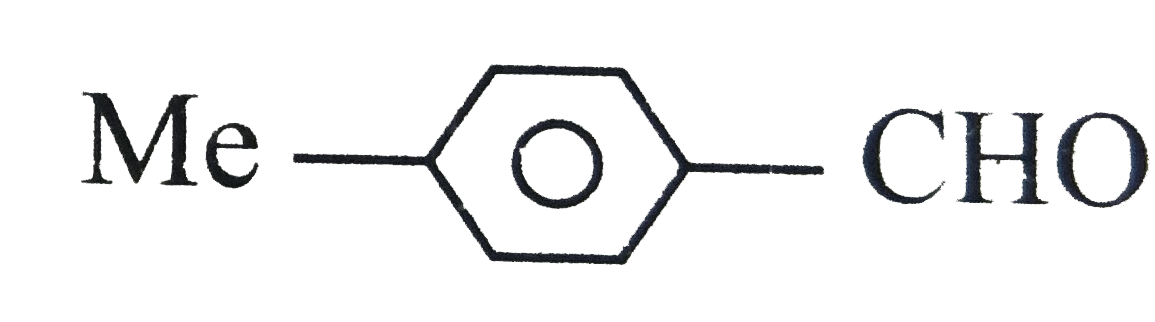
(iii)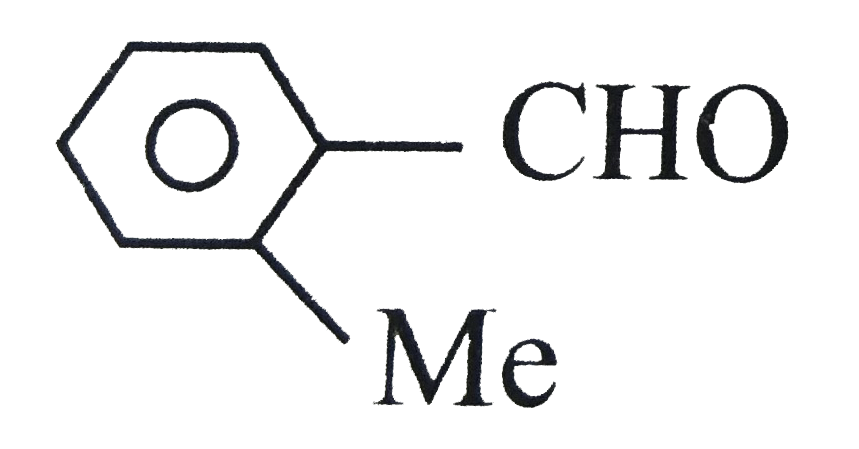
(iv)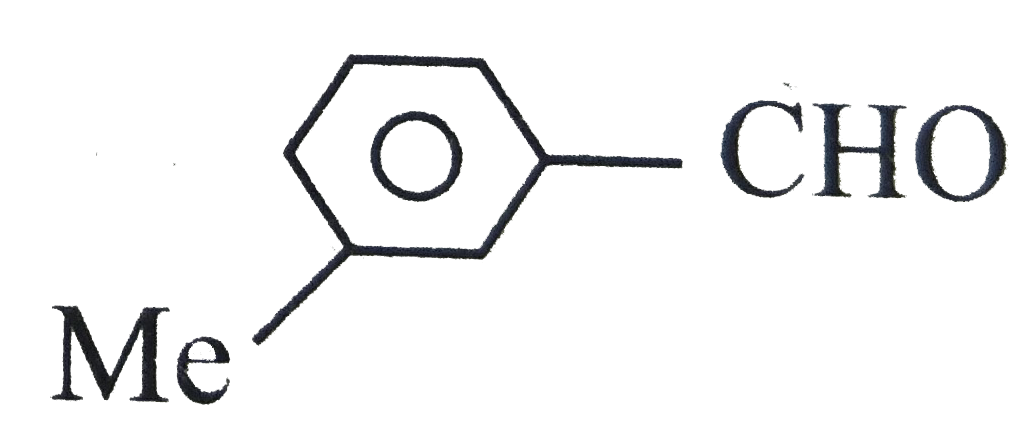 .
.
View Solution

