For a reaction A+2B→C+D, the following data were obtained
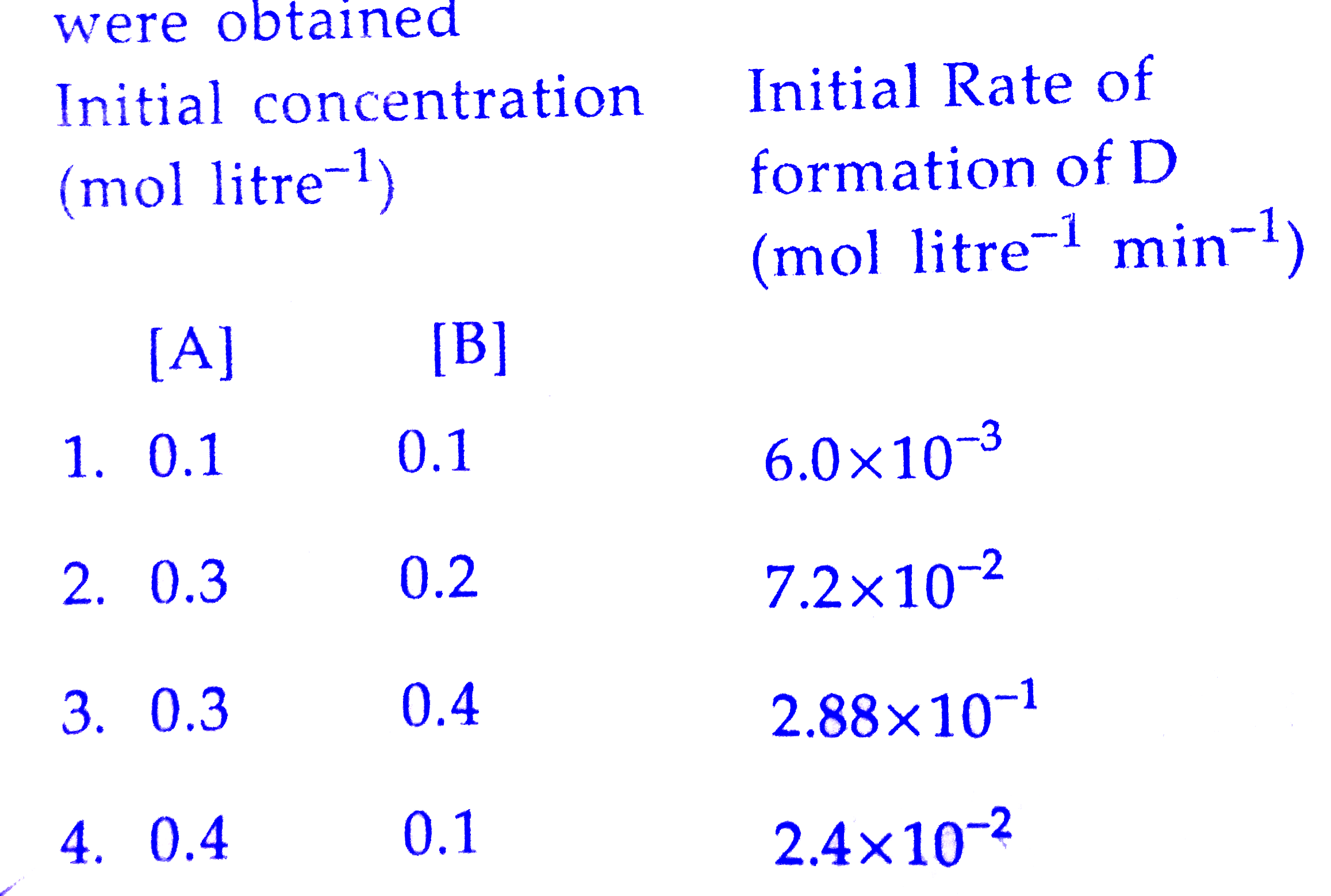
The correct rate law expression will be
Rate =k[A][B]
Rate =k{A][B]2
Rate =k[A]2[B]2
Rate =k[A]2[B]
From 1 and 4, keeping [B] constant, [A] is made 4 times, rate also becomes 4 times
Hence , rate ∝[A].
From 2 and 3, keeping [A] constant [B] is doubled, rate becomes 4 times. Hence,
rate ∝[B]2.
Overall rate law will be rate =k[A][B]2.
Topper's Solved these Questions
Similar Questions
Choose the correct relationship for glucose and fructose
View SolutionFor an endothermic reaction where ΔH represent the enthalpy of reaction in kj/mol, the minimum value for the energy of activation will be:
View SolutionThe following rate data were obtained at 303 K for the following reaction:
2A+B→C+D

What is the rate law? What is the order with respect to each rectant and the overall order? What are the units of the rate constant?
View SolutionThe following initial rate data were obtained at 300K for the reactions: 2A + B to C +D Deducethe rate law.
View SolutionFor the given reaction, A+B→ Products following data were obtained
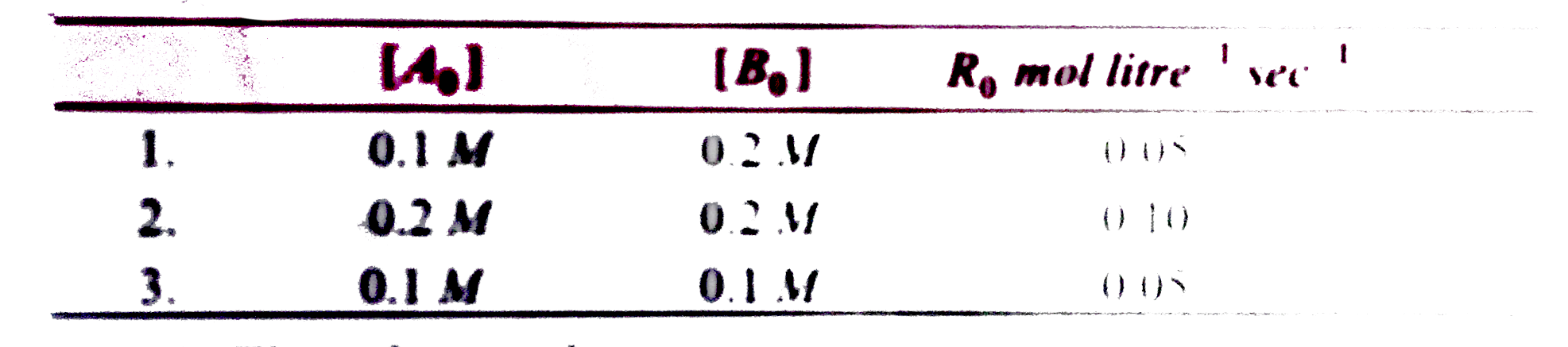
(i) Write the rate law expression.
(ii) Find the rate constant.
View Solutionअभिक्रिया ,A+2B→2C से निम्नलिखित तथ्य प्राप्त हुए-

इस अभिक्रिया के लिए गति सिध्दान्त (Rate law of reaction) स्पष्टीकरण देते हुए लिखे।
View SolutionFor the reaction : A+2B→C+D, the expression of rate of reaction will be :
View SolutionA+2B→C+D for a reaction from following data correct rate law =
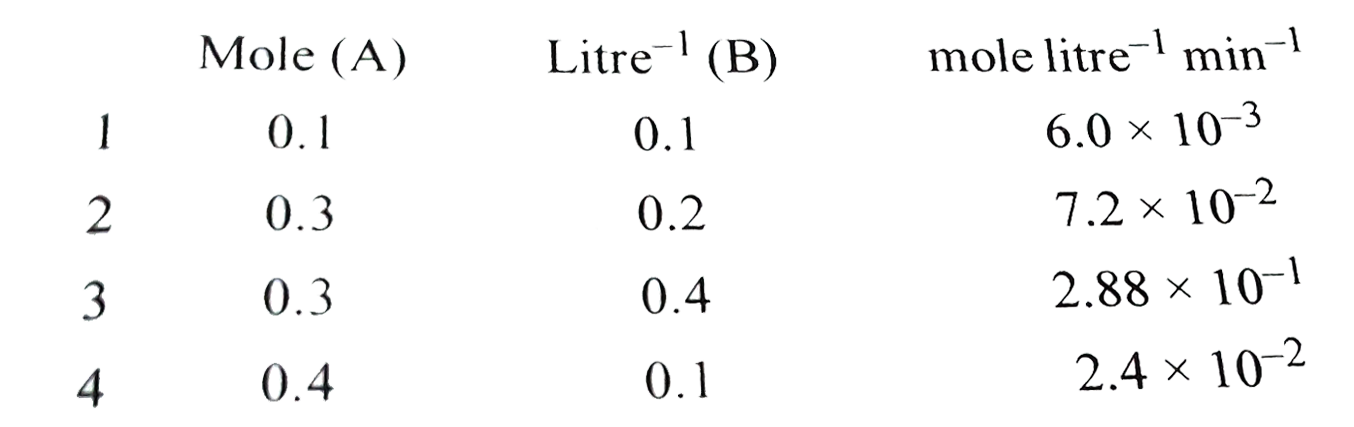
View Solutionरासायनिक रिक्त अभिक्रिया में तीन पृथक् प्रयोगों में 298 K पर निम्न गतिक आँकड़े प्राप्त किये गये।
अभिक्रिया के लिये C बनने की दर नियम होगा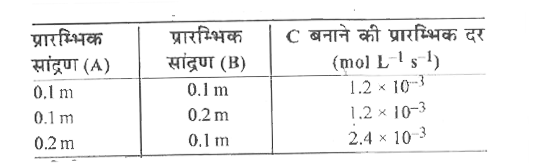
View SolutionThe following rate date were obtained at 313 K for the reaction :
2A+2B→C+D
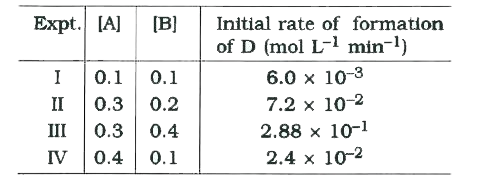
The rate law for the reaction is :
View SolutionFor a reaction A+B→C+2D, experimental results were collected for three trials and the data obtained are given below:
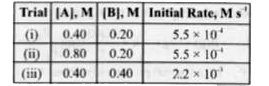
The correct rate law of the reaction is
View SolutionConsider the reaction 2A+2B→2C+D. From the following data, calculate the order and rate constant of the reaction.
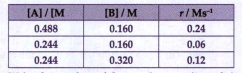
Write the rate law of the reaction.
View SolutionFor the reaction A + 2B to C + D , the following data were obtained: The correct rate law expression will be:
View SolutionFor the reaction : A+2B→C+D, the expression of rate of reaction will be :
View SolutionThe following rate data were obtained at 303 K for the following reaction : 2A +B to C+D What is the rate law? What is the order with respect to each reactant and the overall order? What are the units of rate constant?
View SolutionThe following rate data were obtained at 303 K for the following reaction :
2A+B→C+D

What is the rate law? What is the order with respect to each reactant and the overall order? What are the units of rate constant?
View Solution

