Which of the following molecule do not have the same number of unpaired electron?
d7 octahedral low spin
d5 octahedral low spin
d9 tetrahedral
d6 octahedral low spin
The correct Answer is:D
(a), (b), (C ) have 1 unpaired electron while (d) has 0 unpaired electron.
Topper's Solved these Questions
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:E. Stability, Colour and Magnetic Properties of Complexes
Explore 60 Videos
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:F.Isomerism in Coordination Compounds
Explore 120 Videos
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:C. Werners Theory, EAN Rule
Explore 63 Videos
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS Book:OP TANDONChapter:CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS Exercise:INTEGER
Explore 2 Videos
- ELECTROCHEMISTRYBook:OP TANDONChapter:ELECTROCHEMISTRYExercise:Matrix-Matching Type Question
Explore 3 Videos
Similar Questions
Among the following ions, which one has the highest paramagnetism ?
View SolutionThe complexes [Pt(NH3)4][PtCl6] and [Pt(NH3)4Cl2][PtCl4] are :
View SolutionEAN of a metal carbonyl M(CO)x is 36. If atomic number of metal M is 26, what is the value of x?
View SolutionThe total number of alkyl groups having the molecular formula (c)C3H7(b)C4H9(c)C5H11 are.
View SolutionThis section contains 3 questions. Each question contains statement given in two columns which have to be matched. Statement (a, b, c and d) in Column-I have to be matched with statement (p, q, r and s) in Column-II. The answers to these questions have to be appropriately bubbled as illustrated in the following examples :
If the correct matches are (a-p, s), (b-q, r), (c-p, q) and (d-s), then correct bubbled 4×4 matrix should be as follows:
Match the column-I with Column-II :
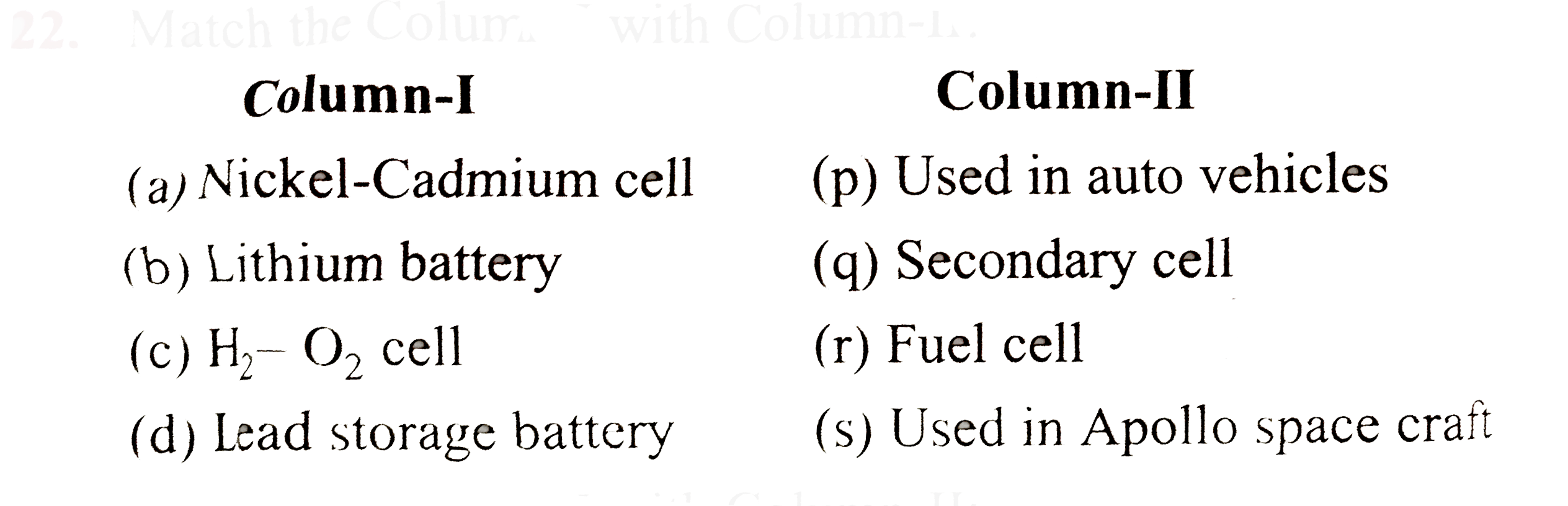
View SolutionWhich of the following have maximum number of unpaired electrons ?
View SolutionPair of ions which are having same number of unpaired electrons
View SolutionWhich of the following have the same number of unpaired electrons in 'd' orbitals
View SolutionWhich of the following pair of ions have the same number of unpaired electrons
View Solutionनिम्नलिखित अणुओं/आयनों में से किसमें अयुग्मित इलेक्ट्रॉन हैं ?
View Solutionनिम्न में से किस अणु/आयन में अयुग्मित इलेक्ट्रॉन नहीं है ?
View SolutionWhich of the following molecule have unpaired
electrons in antibonding molecular orbitals ?
View SolutionWhich of the following molecule do not have the same number of unpaired electron?
View SolutionPair of ions which are having same number of unpaired electrons
View SolutionThe molecule having one unpaired electron is
View Solution

