Among the following pair of complexes, in which case the Δ0 value is higher for the first one?
[Co(NH3)6]3+ and [Co(CN)6]3−
[CoF6]3− and [Co(NH3)6]3+
[Co(H2O)6]2+ and [Co(H2O)6]3+
[Rh(H2O)6]3+ and [Co(H2O)6]3+
The correct Answer is:D
Down the group, C.F. splitting increases 40−50%.
Topper's Solved these Questions
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:E. Stability, Colour and Magnetic Properties of Complexes
Explore 60 Videos
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:F.Isomerism in Coordination Compounds
Explore 120 Videos
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:C. Werners Theory, EAN Rule
Explore 63 Videos
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS Book:OP TANDONChapter:CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS Exercise:INTEGER
Explore 2 Videos
- ELECTROCHEMISTRYBook:OP TANDONChapter:ELECTROCHEMISTRYExercise:Matrix-Matching Type Question
Explore 3 Videos
Similar Questions
Among the following ions, which one has the highest paramagnetism ?
View SolutionThe complexes [Pt(NH3)4][PtCl6] and [Pt(NH3)4Cl2][PtCl4] are :
View SolutionEAN of a metal carbonyl M(CO)x is 36. If atomic number of metal M is 26, what is the value of x?
View SolutionThe total number of alkyl groups having the molecular formula (c)C3H7(b)C4H9(c)C5H11 are.
View SolutionThis section contains 3 questions. Each question contains statement given in two columns which have to be matched. Statement (a, b, c and d) in Column-I have to be matched with statement (p, q, r and s) in Column-II. The answers to these questions have to be appropriately bubbled as illustrated in the following examples :
If the correct matches are (a-p, s), (b-q, r), (c-p, q) and (d-s), then correct bubbled 4×4 matrix should be as follows:
Match the column-I with Column-II :
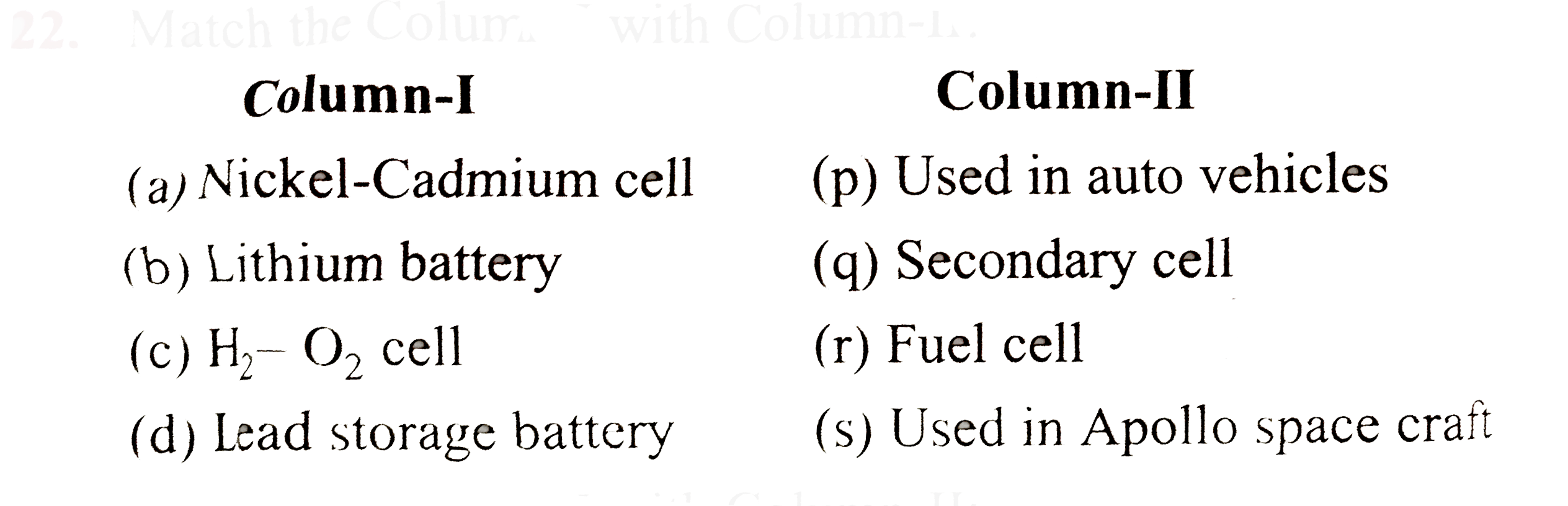
View SolutionWhich of the following complex has higher DeltA_(0) VALUE?
View SolutionThe magnitude of Delta_(0) will be highest in which of the following complex.
View SolutionThe complex having minimum Δ0 value is :
View SolutionWhich of the following complexes has lower Delta_(0) (spliting energy) than pairing energy :-
View SolutionWhich of the following complex has large value of Delta_(0)
View SolutionAmong the following pairs of complexes, in which case the Δ0 value is higher for the first one?
View SolutionIn which of the following octahedral complexes of Co(at. no. 27) , will the magnitude of Delta_(0) be the highest ?
View SolutionSplitting energy (Delta_(0)) can usually be measured form the absorption spectra of the complex ions. In simple cases when light is absorbed by a complex ion, an electron in one of the lower energy orbitals is excited to one of the higher energy orbital. The energy corresponding to the frequency of absorbed light is equal to Delta_(0) . If value of Delta_(0) for the complex is in visible region, the complex is coloured and the value of Delta_(0) lies in ultraviolet or infrared region, the complex is colourless. For octahedral complexes, the cyrstal field stabilisation energy is given by CFSE= +[-0.4t_(2g)N+0.6e_(g)N_(1)]Delta_(0) Where N and N_(1) are number of electrons in t_(2g)" and "e_(g) orbitals respectively. The value of CFSE can be used for the correction of the experimental values of heats of hydration of divalent can be obtained by substracting the calculated CFSE values form the experimental values. Which of the following statements is correct ?
View SolutionAmong the following pair of complexes, in which case the △0 value is higher for the first one?
View SolutionSplitting energy (Δ0) can usually be measured form the absorption spectra of the complex ions. In simple cases when light is absorbed by a complex ion, an electron in one of the lower energy orbitals is excited to one of the higher energy orbital. The energy corresponding to the frequency of absorbed light is equal to Δ0. If value of Δ0 for the complex is in visible region, the complex is coloured and the value of Δ0 lies in ultraviolet or infrared region, the complex is colourless. For octahedral complexes, the cyrstal field stabilisation energy is given by CFSE=+[−0.4t2gN+0.6egN1]Δ0 Where N and N1 are number of electrons in t2g and eg orbitals respectively.
The value of CFSE can be used for the correction of the experimental values of heats of hydration of divalent can be obtained by substracting the calculated CFSE values form the experimental values.
Which of the following statements is correct ?
View Solution

