H2O2 can be produced by the ammonium hydrogen sulphate. Reactions occuring in electrolytic cell,
NH4HSO4→NH−4+2e− (Anode)
2SO2−4→S2O2−8+2e−
2H++2e−→H2 (Cathode)
Hydrolysis of ammonium persulphate:
(NH4)2S2O8+2H2O→2NH4HSO4+H2O2 Assume 100% yield of hydrolysis reaction.
What is the current efficiency when 100 Amp current is passed for 965 seconds in order to produce 17 gm of H2O2.
0.2
0.4
0.8
1
The correct Answer is:D
Step by step video, text & image solution for H_(2)O_(2) can be produced by the ammonium hydrogen sulphate. Reactions occuring in electrolytic cell, NH_(4)HSO_(4)rarrNH_(4)^(-)+2e^(-) (Anode) 2SO_(4)^(2-)rarrS_(2)O_(8)^(2-)+2e^(-) 2H^(+)+2e^(-)rarrH_(2) (Cathode) Hydrolysis of ammonium persulphate: (NH_(4))_(2)S_(2)O_(8)+2H_(2)Orarr2NH_(4)HSO_(4)+H_(2)O_(2) Assume 100% yield of hydrolysis reaction. What is the current efficiency when 100 Amp current is passed for 965 seconds in order to produce 17 gm of H_(2)O_(2). by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ELECTROCHEMISTYBook:GRB PUBLICATIONChapter:ELECTROCHEMISTYExercise:Match the column type
Explore 16 Videos
- ELECTROCHEMISTYBook:GRB PUBLICATIONChapter:ELECTROCHEMISTYExercise:Subjective type
Explore 39 Videos
- ELECTROCHEMISTYBook:GRB PUBLICATIONChapter:ELECTROCHEMISTYExercise:Multiple objective type
Explore 33 Videos
- D-BLOCK ELEMENTSBook:GRB PUBLICATIONChapter:D-BLOCK ELEMENTSExercise:Subjective Type
Explore 18 Videos
- ENVIRONMENTAL CHEMISTRY Book:GRB PUBLICATIONChapter:ENVIRONMENTAL CHEMISTRY Exercise:Straight objective type
Explore 40 Videos
Similar Questions
Mathc the following columns :
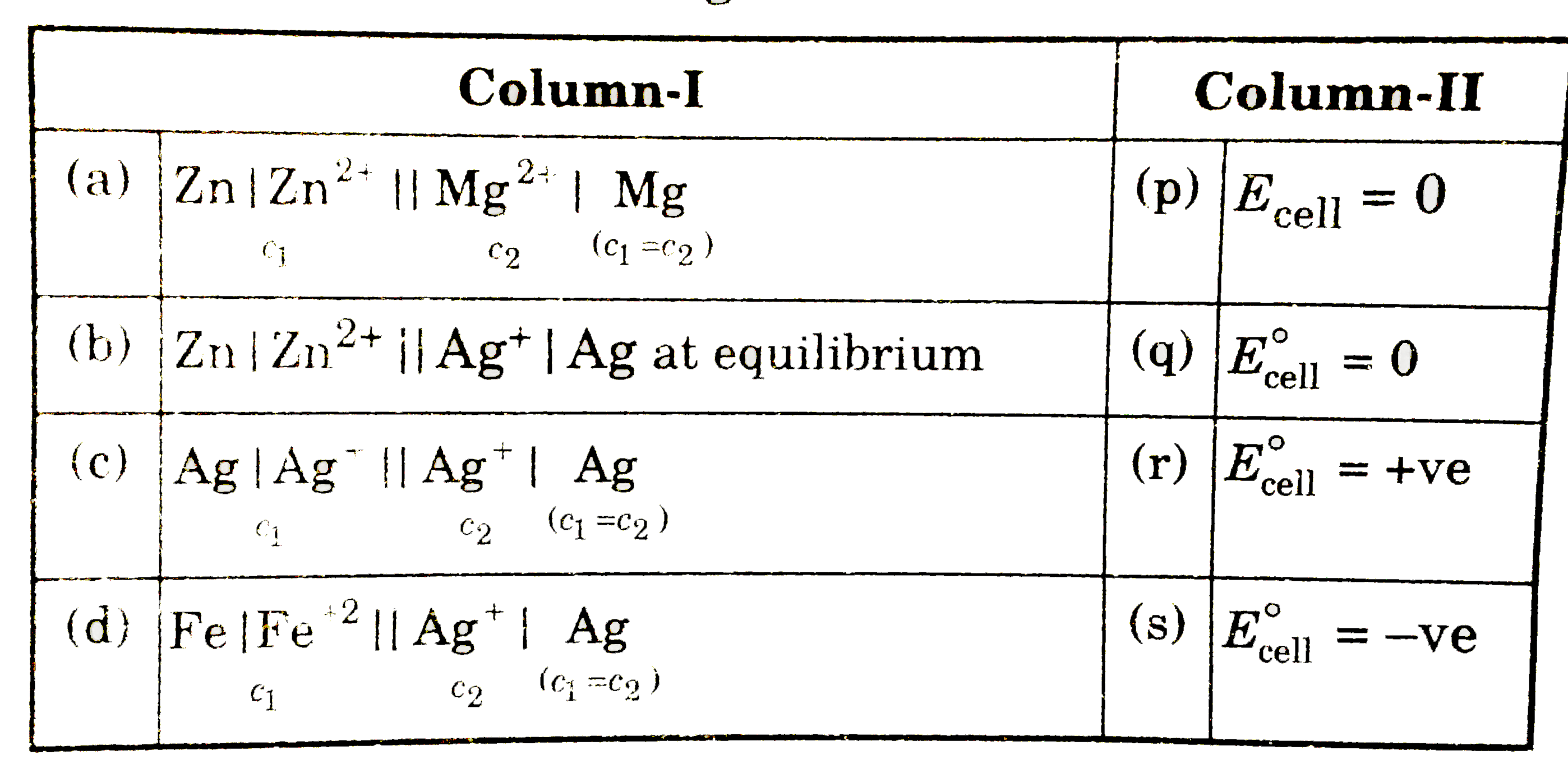
View SolutionThe conductivity ofa solution may be taken to be directly proportional to the total concentration of the charge carriers (ions) present in it in many cases. Using the above, find the percent decrease in conductivity (k) of a solution of a week monoacidic base BOH when its 0.1 M solution is diluted to double its original volume. (Kb=10−5forBOH)(takesqrt(50) = 7.07) (mark the answer to nearest integer)
View SolutionWhich of the following statements is wrong about galvanic cells?
View SolutionNumber of halide ions among (F−,Cl−,Br−,I−) which change their oxidation number on heating with MnO2+conc.H2CO4
View SolutionWhich of the following cannot be degraded by natural processes ?
(P) DDT , (Q) Plastic materials
(R) Heavy metals , (S) Nuclear wastes
View SolutionH2O2 can be produced by the Ammonium hydrogen sulphate. Reactions occuring in electrolytic cell.
NH4HSO4→NH+4+H++SO2−4
2SO2−4→S2O2−8+2e− (Anode)
2H++2e−→H2 (cathode)
(NH4)2S2O8+2H2O→2NH4HSO4+H2O2
Hydrolysis of Ammonium persulphate
Assume 100% yield of hydrolysis reaction.
What is the current efficiency when 100 Amp current is passed for 965 sec, in order to produce 17 gm of H2O2.
View SolutionH2O2 can be produced by the Ammonium hydrogen sulphate. Reactions occuring in electrolytic cell.
NH4HSO4→NH+4+H++SO2−4
2SO2−4→S2O2−8+2e− (Anode)
2H++2e−→H2 (cathode)
(NH4)2S2O8+2H2O→2NH4HSO4+H2O2
Hydrolysis of Ammonium persulphate
Assume 100% yield of hydrolysis reaction.
How many moles of electrons are to be passed in order to produce enough H2O2 which when reacted with excess of KI then liberated iodine required 100 ml of centimolar Hypo solution.
View SolutionHydrogen peroxide can be prepared by successive reaction: 2NH_(4)HSO_(4) rarr H_(2)+(NH_(4))_(2)S_(2)O_(8) (NH_(4))_(2)S_(2)O_(8)+2H_(2)O rarr 2NH_(4)HSO_(4)+H_(2)O_(2) The first reaction is an electrolytic reaction the second is steam distillation. what amount ofcurrent would have to be used in first reaction to produce enough intermediate to yield 100g pure H_(2)O_(2) per hour ? Assume 50% anode current efficiency.
View SolutionH2O2 can be produced by the ammonium hydrogen sulphate. Reactions occuring in electrolytic cell,
NH4HSO4→NH−4+2e− (Anode)
2SO2−4→S2O2−8+2e−
2H++2e−→H2 (Cathode)
Hydrolysis of ammonium persulphate:
(NH4)2S2O8+2H2O→2NH4HSO4+H2O2 Assume 100% yield of hydrolysis reaction.
What volume of hydrogen gas at 1 atm and 273 K will be produced in cathode in previous question?
View SolutionH_(2)O_(2) can be produced by the ammonium hydrogen sulphate. Reactions occuring in electrolytic cell, NH_(4)HSO_(4)rarrNH_(4)^(-)+2e^(-) (Anode) 2SO_(4)^(2-)rarrS_(2)O_(8)^(2-)+2e^(-) 2H^(+)+2e^(-)rarrH_(2) (Cathode) Hydrolysis of ammonium persulphate: (NH_(4))_(2)S_(2)O_(8)+2H_(2)Orarr2NH_(4)HSO_(4)+H_(2)O_(2) Assume 100% yield of hydrolysis reaction. How many "mole"s of electrons are to be passed in order to produce enough H_(2)O_(2) which when reacted with excess of KI then liberated iodine required 100 mL of centimolar hypo solution.
View SolutionAnodic oxidation of ammonium hydrogen sulphate produces ammonium persulphate. NH_(4) HSO_(4) rarr NH_(4)SO_(4)^(-) + H^(+) 2NH_(4) SO_(4)^(-) rarr (NH_(4))_(2) S_(2) O_(8) + 2e^(-) (Anodic oxidation) 2H^(+) + 2e^(-) rarr H_(2) (Cathodic reduction) Hydrolysis of ammonium persulphate forms H_(2)O_(2) (NH_(2))_(2) S_(2)O_(8) + 2H_(2)O rarr 2NH_(4) HSO_(4) + H_(2)O_(2) Current efficiency in electrolytic process is 60%. Calculate the amount of current required to produce 85 g of H_(2)O_(2) per hour: Hydrolysis reaction shows 100% yield.
View SolutionDuring an electrolysis of conc. H2SO4 perdisulphuric acid (H2S2O6) and O2 form in equimolar amount. The amount of H2 than will form simultaneously at other electrode will be (2H2SO4→H2S2O8+2H++2e−)
View Solutionनिम्न किन अभिक्रयाओं में H2O2 एक अपचायक का काम करता है ?
(1) H2O2+2H+2e−→2H2O
(2) H2O2−2e−→O2+2H+
H2O2+2e−→2OH−
(4) H2O2+2OH−−2e−→O2+2H2O
View SolutionIn which of the following reactions, H_(2)O_(2) acts as a reducing agent- (1) H_(2)O_(2) + 2H^(+) + 2e rarr 2H_(2) O (2) H_(2)O_(2) - 2e rarr O_(2) + 2H^(+) (3) H_(2)O_(2) + 2e rarr 2OH^(-) (4) H_(2)O_(2) + 2H^(-) - 2e rarr O_(2) + 2H_(2) O
View Solutionनिम्न अभिक्रिया को पूर्ण तथा संतुलित कीजिए
(NH4)2S2O8+H2O+MnSO4→…+…+…
View Solution
