An element (X) having same cheical properties as that of hydrogen atom. The diatomic "mole"cule X2 is gaseous in nature. The energy required to remove an electron from the outermost shell of (X) atom is 13.5eV. It is also found that the time taken for diffusion of equal volume of gaseous X2 and O2 at the same pressure is in the ratio √3:4. the normal freezing point of pure X2O is 0∘C. However, on adding 0.02 "mole"s of a non-electrolyte solute to 0.8 "mole"s of X2O, the freezing point of solution is found to be-1.25∘C. X-atom also obey Bohr's model.
What will be the energy required to excite 5 (X) atoms to second exicted state?
30eV
60eV
120eV
15eV
The correct Answer is:B
Topper's Solved these Questions
- ELECTROCHEMISTYBook:GRB PUBLICATIONChapter:ELECTROCHEMISTYExercise:Match the column type
Explore 16 Videos
- ELECTROCHEMISTYBook:GRB PUBLICATIONChapter:ELECTROCHEMISTYExercise:Subjective type
Explore 39 Videos
- ELECTROCHEMISTYBook:GRB PUBLICATIONChapter:ELECTROCHEMISTYExercise:Multiple objective type
Explore 33 Videos
- D-BLOCK ELEMENTSBook:GRB PUBLICATIONChapter:D-BLOCK ELEMENTSExercise:Subjective Type
Explore 18 Videos
- ENVIRONMENTAL CHEMISTRY Book:GRB PUBLICATIONChapter:ENVIRONMENTAL CHEMISTRY Exercise:Straight objective type
Explore 40 Videos
Similar Questions
Mathc the following columns :
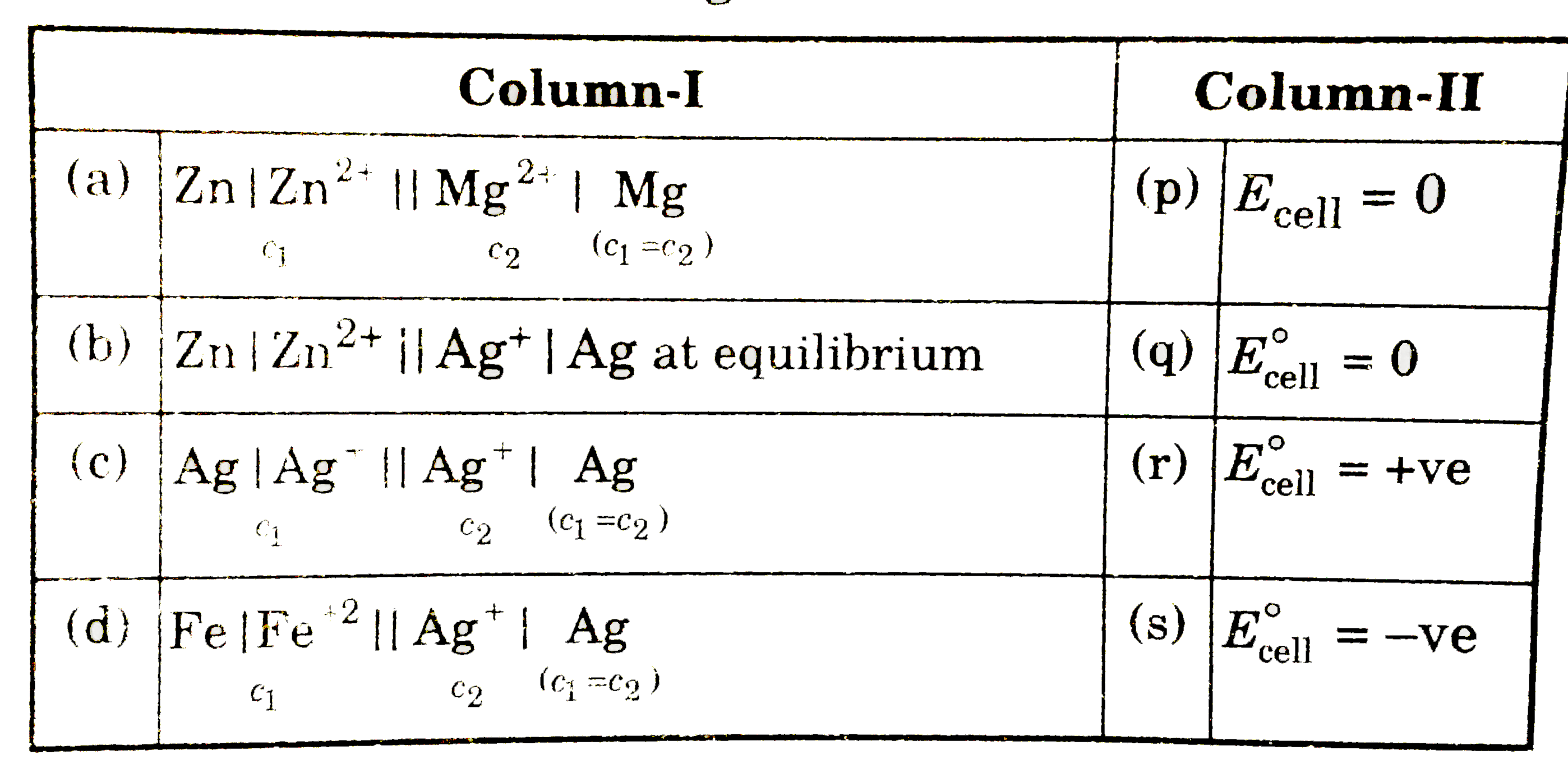
View SolutionThe conductivity ofa solution may be taken to be directly proportional to the total concentration of the charge carriers (ions) present in it in many cases. Using the above, find the percent decrease in conductivity (k) of a solution of a week monoacidic base BOH when its 0.1 M solution is diluted to double its original volume. (Kb=10−5forBOH)(takesqrt(50) = 7.07) (mark the answer to nearest integer)
View SolutionWhich of the following statements is wrong about galvanic cells?
View SolutionNumber of halide ions among (F−,Cl−,Br−,I−) which change their oxidation number on heating with MnO2+conc.H2CO4
View SolutionWhich of the following cannot be degraded by natural processes ?
(P) DDT , (Q) Plastic materials
(R) Heavy metals , (S) Nuclear wastes
View SolutionIf x_(1),x_(2),x_(3) as well as y_(1),y_(2),y_(3) are also in G.P. With the same common ratio,then the points (x_(1),y_(1)),(x_(2),y_(2)),(x_(3),y_(3)) lies on
View SolutionIf X_(M) , X_(P) , and X_(V) are mole fraction, pressure fraction and volume fraction respectively of a gaseous mixture, then:
View SolutionN_(0)//2 atoms of X_((g)) are converted into X_((g))^(o+) by energy E_(1), N_(0)//2 atoms of X_((g)) are converted inot X_((g))^(ɵ) by energy E_(2) . Hence ionisation potential and electron affinity of X_((g)) per atom are
View SolutionIf X_(M),X_(P) and X_(V) are mole fration, perssure fraction and volume fraction respectively of a gaseous component in a mixture then .
View SolutionAn isotope of hydrogen atom is represented as X which follows Bohr's model and exists as diatomic gaseous molecule X_(2) . Also the normal boiling point of a compound X_(2)O liquid is found to be 101^(@) C and that of solution obtaind on dissolving 0.1 moles of NaCl in 1 kg of X_(2)O liquid is 101.4^(@)C . It is also known that the ionization energy of X is equal to 14 eV . The energy required to excite electron from ground state of IInd excited state is given by :
View SolutionAn element (X) having same cheical properties as that of hydrogen atom. The diatomic "mole"cule X_(2) is gaseous in nature. The energy required to remove an electron from the outermost shell of (X) atom is 13.5eV. It is also found that the time taken for diffusion of equal volume of gaseous X_(2) and O_(2) at the same pressure is in the ratio sqrt(3):4 . the normal freezing point of pure X_(2)O is 0^(@)C . However, on adding 0.02 "mole"s of a non-electrolyte solute to 0.8 "mole"s of X_(2)O , the freezing point of solution is found to be- 1.25^(@)C . X-atom also obey Bohr's model. When 3 gm of a weak monoprotic acid (M_(w)=60) is added to 0.8 "mole"s of X_(2)O , the resulting solution freeze at -3.75^(@) C. What is the degree of dissociation for weak acid?
View SolutionAn element (X) having same cheical properties as that of hydrogen atom. The diatomic "mole"cule X2 is gaseous in nature. The energy required to remove an electron from the outermost shell of (X) atom is 13.5eV. It is also found that the time taken for diffusion of equal volume of gaseous X2 and O2 at the same pressure is in the ratio √3:4. the normal freezing point of pure X2O is 0∘C. However, on adding 0.02 "mole"s of a non-electrolyte solute to 0.8 "mole"s of X2O, the freezing point of solution is found to be-1.25∘C. X-atom also obey Bohr's model.
What amount of electricity is required to produce 3.3 gm of x2 gas by electrolysis of X2O?
View SolutionLet the equation x5+x3+x2+2=0 has roots x1,x2,x3,x4andx5, then find the value of (x22−1)(x23−1)(x24−1)(x25−1).
View Solutionयदि 10 प्राकृत संख्याओं x1,x2,x3,…………x10 का माध्य ¯¯¯X हो, तो प्रदर्शित करो कि :
(x1−¯¯¯X)+(x2−¯¯¯X)+…………..+(x10−¯¯¯X)=0
View SolutionIf A=[−1342],B=[213−5],X=[x1x2x3x4] and A+B=X.
then find the values of x1,x2,x3,x4.
View Solution

