For a certain van der Waal's gas, critical temperature is-243∘C. Match the graphs (given in column-I) with the temperature of the gas (given in column-II).
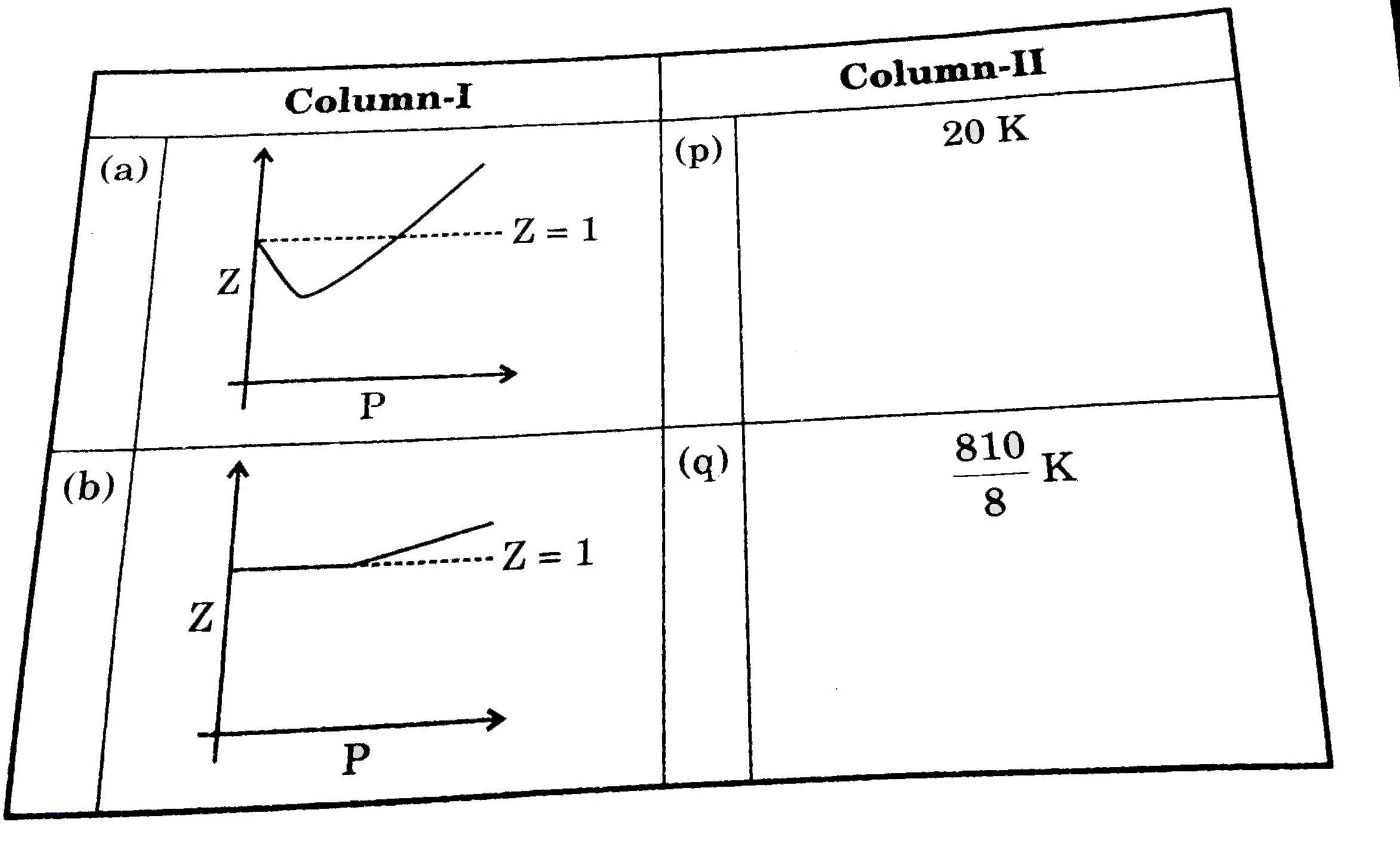
Text Solution
The correct Answer is:a-p,s ; b-q; c-r
|
Topper's Solved these Questions
- GRAPHICAL INTERPRETATIONBook:GRB PUBLICATIONChapter:GRAPHICAL INTERPRETATIONExercise:Subjective Type
Explore 23 Videos
- GRAPHICAL INTERPRETATIONBook:GRB PUBLICATIONChapter:GRAPHICAL INTERPRETATIONExercise:Comprehension 29
Explore 1 Video
- F-BLOCK ELEMENTSBook:GRB PUBLICATIONChapter:F-BLOCK ELEMENTSExercise:Subjective type
Explore 7 Videos
- HYDROCARBON (ALIPHATIC)Book:GRB PUBLICATIONChapter:HYDROCARBON (ALIPHATIC)Exercise:Previous years jee questions
Explore 28 Videos
Similar Questions
Knowledge Check
For a real gas (van der Waal's gas)
ABoyle temperature is a /RbBCritical temperature is a /RbCTriple temperture is a 2a/RbDInversions temperature is a /2RbFor real gases (van der waals ' gas )
ABoyle temperature is a /2RBCritical temperature is 8a /27RbCTriple temperture is a 2a/2bDInversions temperature is a /2RbThe van der Waal's gas constant, a is given by
A3VcB27b2PcC27RbTcD3RbPc
For a gas obeying the van der Waals' equation, at the critical point
View SolutionThe van der Waal's gas constant 'a' is given by:
View SolutionFor a gas obeying the van der Waals' equation, at the critical point
View SolutionThe van der Waals' equation for a real gas is:
View SolutionCompressibility factor (Z) for a van der Waals real gas at critical point is
View Solution