For adsorption of a gas on solid surface, following isotherm was obtained. Where P is pressure of gas in atm, x represents amount of gas adsorbed and m represents mass of adsorbent, calculate the value of xm of the gas at same temperature and at a pressure of 81 atm.
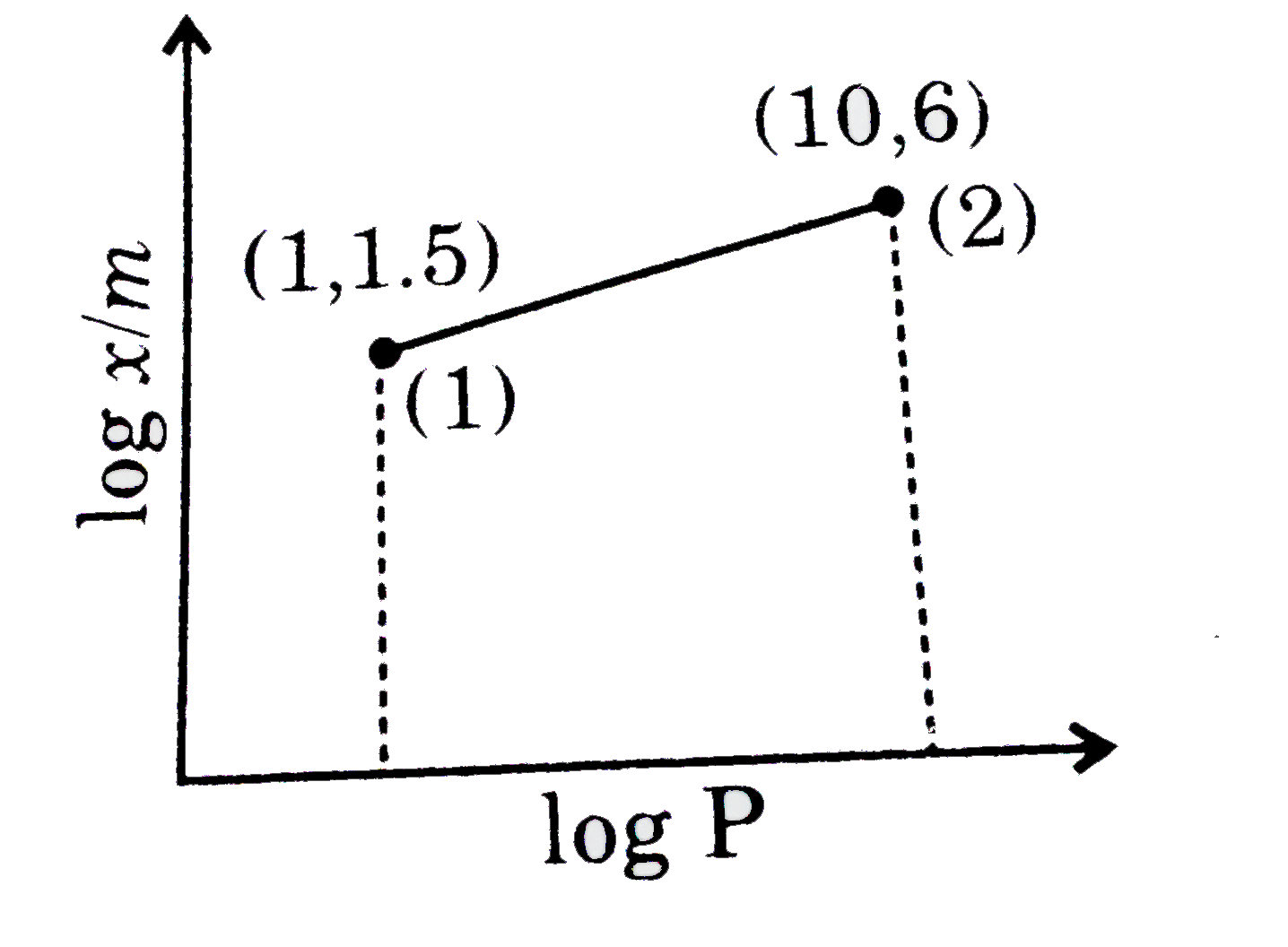
The correct Answer is:90
Topper's Solved these Questions
- GRAPHICAL INTERPRETATIONBOOK - GRB PUBLICATIONCHAPTER - GRAPHICAL INTERPRETATIONEXERCISE - Match The Column Type
15 Videos
- F-BLOCK ELEMENTSBOOK - GRB PUBLICATIONCHAPTER - F-BLOCK ELEMENTSEXERCISE - Subjective type
7 Videos
- HYDROCARBON (ALIPHATIC)BOOK - GRB PUBLICATIONCHAPTER - HYDROCARBON (ALIPHATIC)EXERCISE - Previous years jee questions
28 Videos
Similar Questions
At a temperature of 180 K, O2 gas adsorbs on Pt surface following Freundlich adsorption isotherm. Following experimental data is obtained.
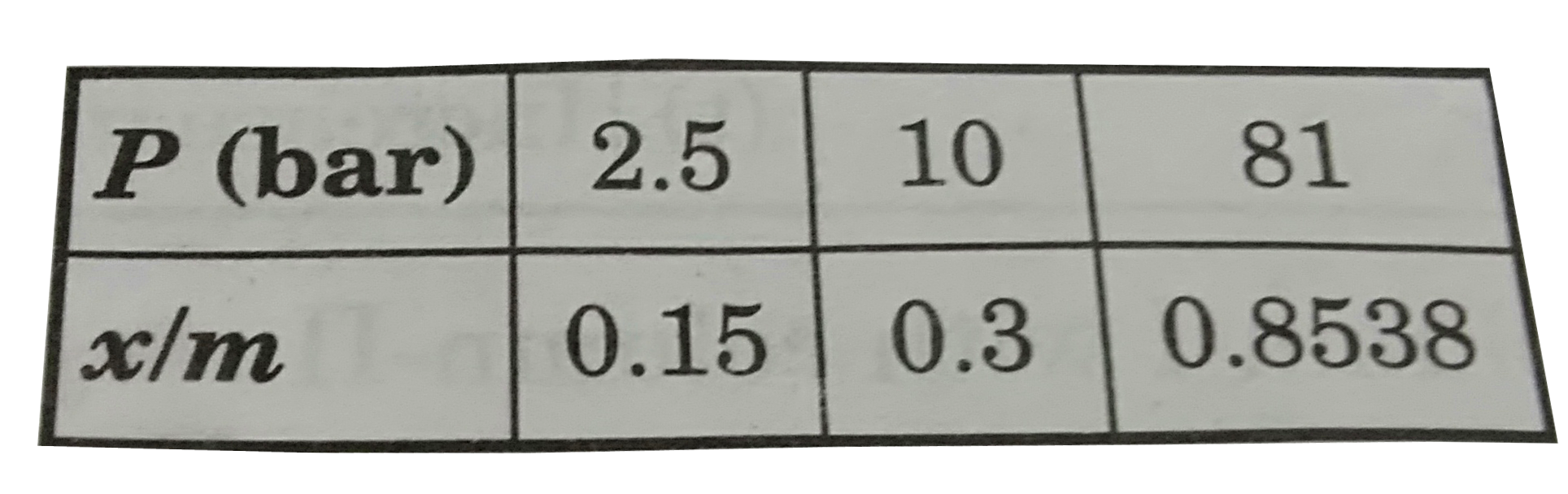
where P is partial pressure of gas and x/m is mass of gas adsorbed per gm of platinum. Calculate millimoles of O2 adsorbed per gm of platinum at 4 bar pressure and 180 K.
View SolutionIn plot of mass of the gas adsorbed per gram of the adsorbent versus temperature at constant pressure is called ………..
View Solution
Knowledge Check
The adsorption of gas on a solid surface varies with pressure of the gas in which of the following manner.
Afast to slow to independent of the pressureBslow to fast to independent of the pressureCindependent of the pressure to fast to slowDindependent of the pressure to slow to fastThe adsorption of gas on a solid surface varies with pressure of the gas in which of the following manner.
Afast → slow → independent of the pressureBslow → fast → independent of the pressureCIndependent of the pressure → fast → slowDIndependent of the pressure → slow → fastBased on Langmuir adsorption isotherm ,at very high pressures the amount of gas adsorbed
AIncreases first and decreases later with pressureBReaches a constant limiting valueCGoes on decreasing with pressureDGoes on increasing with pressure
According of Langmuir adsorption isotherm amount of gas adsorbed at very low pressure
View SolutionA graph between the amount of gas adsorbed per gram of the absorbent and equilibrium pressure of the adsorbate at constant temperature is called ................. .
View SolutionIn which of the following manner the adsorption of a gas on a solid surface varies with pressure of the gas?
View SolutionAccording of Langmuir adsorption isotherm amount of gas adsorbed at very low pressure
View SolutionAdsorption of a gas follows Freundlich adsorption isotherm. In the given plot,x is the mass of the gas adsorbed on mass m of the adsorbent at pressure P. xm is proportional to:
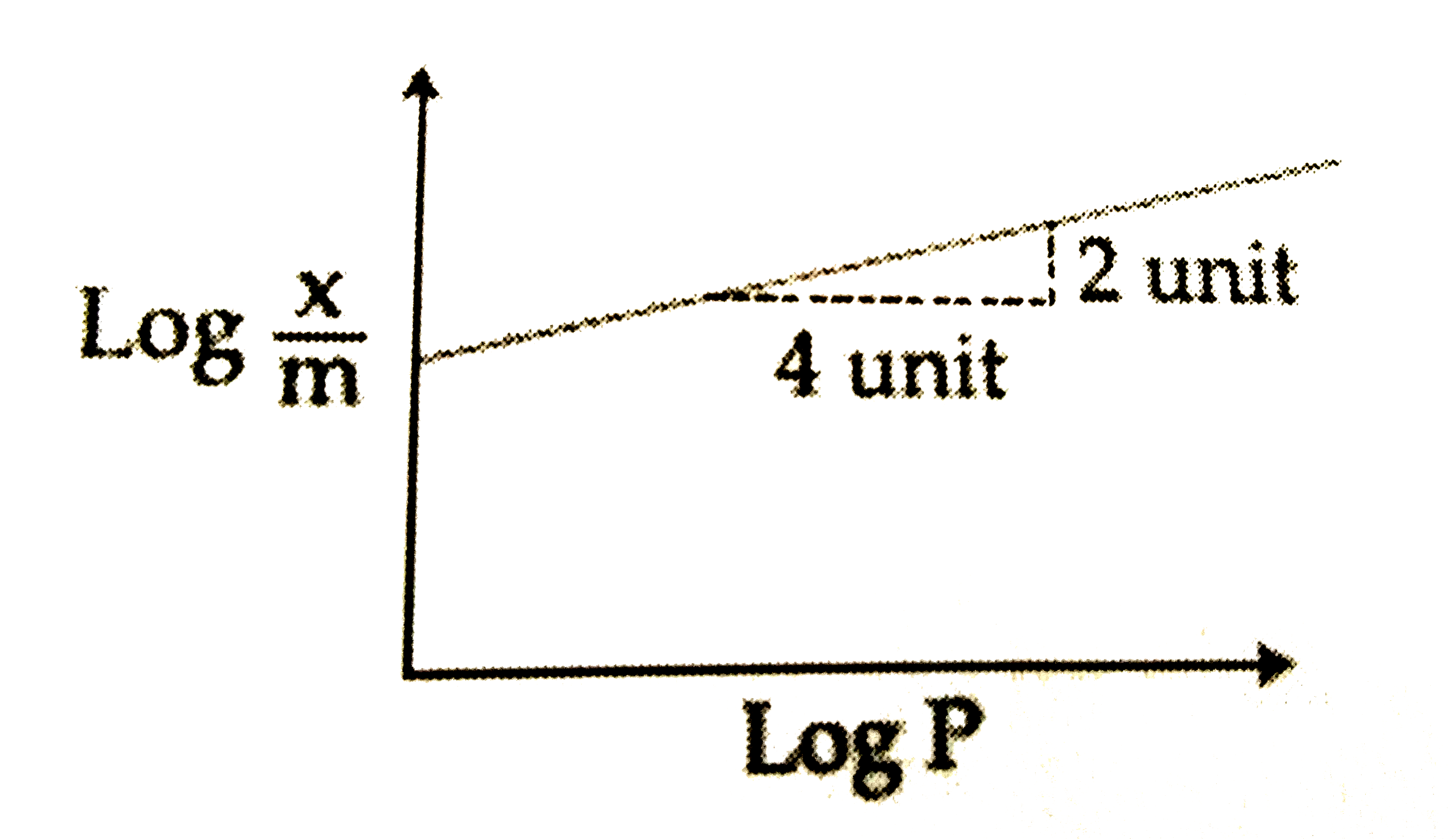
View Solution

