Compound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
What could be the structure of compound (A) if neither dissolves in aq. NaHCO3 nor gives a characteristic colour with FeCl3?
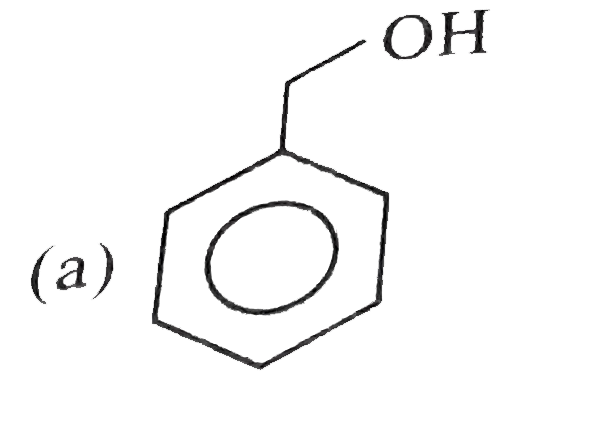
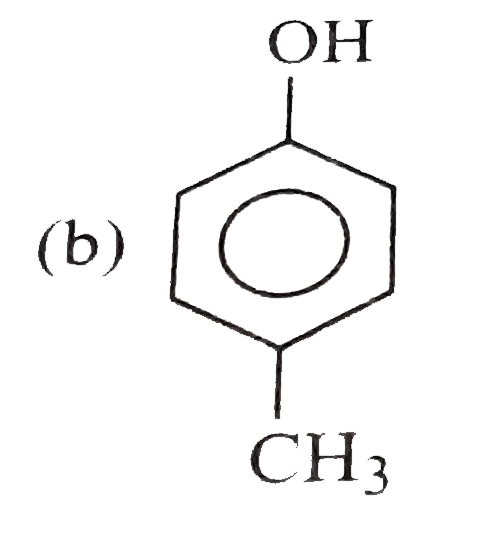
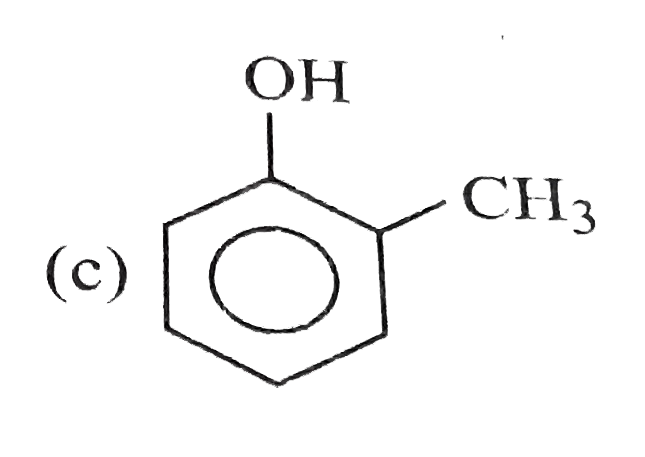
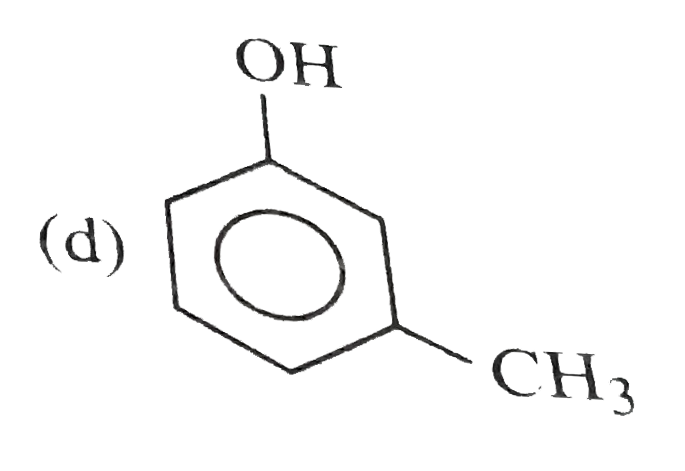
The correct Answer is:A, B
Step by step video, text & image solution for Compound (A)C_(7)H_(8)O is insoluble in aqueous NaHCO_(3) and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl_(3) . When treated with Br_(2)(A) forms compound (B) C_(7)H_(5)O Br_(3). What could be the structure of compound (A) if neither dissolves in aq. NaHCO_(3) nor gives a characteristic colour with FeCl_(3)? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- PRACTICAL ORGANIC CHEMISTRYBOOK - HIMANSHU PANDEYCHAPTER - PRACTICAL ORGANIC CHEMISTRYEXERCISE - Match The Column
6 Videos
- PRACTICAL ORGANIC CHEMISTRYBOOK - HIMANSHU PANDEYCHAPTER - PRACTICAL ORGANIC CHEMISTRYEXERCISE - More Than One Correct
15 Videos
- POLYMERSBOOK - HIMANSHU PANDEYCHAPTER - POLYMERSEXERCISE - Integer Answer Type Problems
8 Videos
Similar Questions
Column(I)Column(II)(a)Ph−O∣∣C−CH3P.Aldol condensation(b)CH3−O∣∣C−HQ.Positive iodoform test(c)Ph−O∣∣C−HR.Negative test of Fehling's solution(d)CH3CH2OHS.Oxidation withCu/Δ
View SolutionWhich of the following aromatic compounds will react with KMnO4?
View SolutionThe number of condensation copolymers among the following is_________.
SBR, polyester, bakelite, nylon-6, PVC, starch, nylon-6,6, glyptal, natural rubber.
View SolutionAn organic compound (A) with molecular formula C_(7)H_(8)O dissolves in NaOH and gives characteristic colour with FeCI_(3) . On treatement with Br_(3) , it gives a tribromo product C_(7)H_(5)Br_(3) . The compound is:
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The most probable structure of compound A is :
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The structure of compound (B) would be :
View SolutionCompound A (C7H8O) is insoluble in water , dilute HCL and aqueous NaHCO3 it dissolves is dilute NaOH. When A is treated with Br2 water it is converted into a compound C7H5Obr3 reapidly . The structure of A is :
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The most probable structure of compound A is :
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The structure of compound (B) would be :
View SolutionA compound 'A' (C7H8O) is insoluble in water, dilute HCl and aqueous NaHCO3 but soluble in dilute NaOH . When A is treated with Br2−H2O, it is converted into a compound of formula C7H5Obr3. Compound A is :
View SolutionCompound A (C7H8O) is insoluble in water, dilute HCl & aqueous NaHCO3, but it dissolves in dilute NaOH. When A is treated with Br2 water it is converted into a compound C7H5Obr3 rapidly. The structure of A is:
View SolutionAn organic compound with molecular formula C7H8O dissolves in NaOH and gives characteristic colour with FeCl3. On treatment with Br2 it gives tribromo product C7H5OBr3. The compound is
View SolutionAn organic compound [X] of the formula C7H8O is soluble in NaOH but not in NaHCO3. It gives colour with alcoholic FeCl3. On treatment with bromine water it gives a tribromo product. The compound (A) is :
View SolutionAn organic compound (A) C_(7)H_(8)O is insoluble in aqueous NaHCO_(3) , but soluble in NaOH. (A) on treatment with bromine water rapidly forms compound (B), C_(7)H_(5)Obr_(3) . The compound (A) is ....
View SolutionAn organic compound (A) with molecular formula C_(7)H_(8)O dissolves in NaOH and gives characteristic colour with FeCl_(3) . On treatement with Br_(3) , it gives a tribromo product C_(7)H_(5)Br_(3) . The compound is:
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The most probable structure of compound A is :
View Solution

