Discuss in detail about the variation of internal energy with respect to variation thermodynamic processes.
Mathematics statement of the first law of thermodynamics is
ΔU=q+w
Case 1 : For a cyclic process involving isothermal expansion of an ideal gas
ΔU=0,∴Q=−w
In other words, during a cyclic process , the amount of heat absorbed by the system in equal to work done by the system.
Case 2 : For an isolated process ( no change in volume ) there is no work of expansion.
ΔV=0,w=0,ΔU=qv
In other words , during isochoric process , the amount of heat supplied to the system is converted fl to its internal energy .
Case 3 : For an adiabatic process there is no change in heat i.e. q = 0 . Hence
q=0,ΔU=w
In other words, in a adiabatic process , the decrease in internet energy is exactly equal to the work done by the system on its surroundings.
in the pressure . P remains constant . Hence
ΔU=q+w
ΔU=q−PΔV
n other words , in an isolated process a part of heat absorbed by the system is used for PV expansion work and the remaining is added to the internal energy of the system.
Similar Questions
Knowledge Check
If ΔU and ΔW represent the increase in internal energy and work done by the system resectively in a thermodynamical process, which of the following is true?
AΔU=−ΔW , in a isothermal processBΔU=−ΔW in a adiabatic processCΔU=ΔW , in a isothermal processDΔU=ΔW , in a adiabatic processIf ΔU and ΔW represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true ?
AΔU=−ΔW, in a adiabatic processBΔU=ΔW, in isothermal processCΔU=ΔW, in a adiabatic processDΔU=−ΔW, in a isothermal process
Thermodynamics - Internal Energy
View SolutionDiscuss the variation of g with altitude.
View SolutionDiscuss the variation in the animals regarding the excretory product ?
View SolutionFigure shows the variation of the internal energy U with the density ρ of an ideal monoatomic gas for a therodynamic process AB. Process AB is a part of arectangular hyperbola. Find the work done (in joule) by gas in process AB.
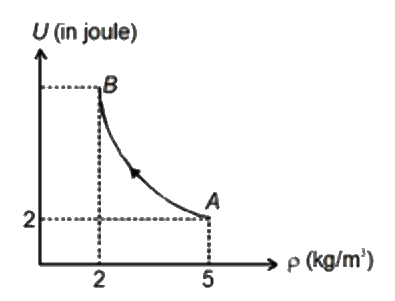
View SolutionFigure shows the variation of the internal energy U with the density ρ of an ideal monoatomic gas for a therodynamic process AB. Process AB is a part of arectangular hyperbola. Find the work done (in joule) by gas in process AB.

View SolutionFigure shows the variations of the internal energy U With density ρ one mole an ideal monatomic gas for thermodynamic cycle ABCA . Here process AB is a part of rectangular hyperbola,-
_S01_092_Q01.png)
View Solution

