Explain the hybridisation, geometry and magnetic prpoperty of [Co(NH3)6]3+ based on VBT.Solution in Kannada
(i) Orbitals of Co3+ ion 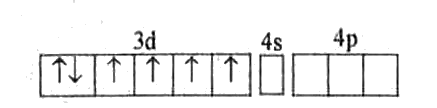
(ii) In presence of strong ligand like NH3 electrons pairing takes place and d2sp3 hybridisation takes place.
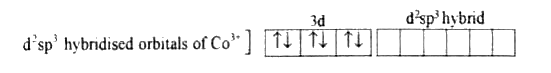
(iii) Six pairs of electrons, one from each NH3 molecule occupy the six hybrid orbitals, then the complex has the octahedral geometry and is diamagnetic because of the absence of unpaired electrons.
[Co(NH3)6]3+ complex = 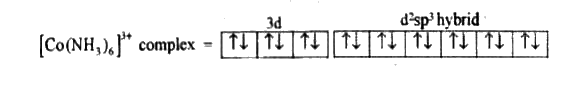
For [CO(NH3)4]3+ Complex :
(i) Type of hybridisation : d2sp3
(ii) Geometry : Octahedral
(iii) Magnetic property : Diamagnetic.
Similar Questions
Using VBT, explain the geometry and magnetic property of [CO(NH_(3))_(6)]^(+3) .
View SolutionWith the help of Valence Bond Theory (VBT) explain hybridisation, geometry and magnetic property of [CoF_6]^2 heat fluoridocobaltate (III) ion.
View SolutionUsing VBT, explain the geometry and magnetic property of [CO(NH3)6]+3.
View SolutionWith the help of valence bond theory account for the geometry and magnetic property of (Co(NH3)6)3+.
View SolutionUsing VB theory, explain the type of hybridisation , geometry , magnetic property and magnetic moment of [Fe(CN)_(6)]^(3-)
View SolutionExplain the hybridisation, geometry, magentic property and magnetic moment of [COF_(6)]^(3-)
View SolutionWhat scheme of hybridisation is proposed for Co in [Co(NH3)6]3+ ?
View SolutionUsing valence bond theory explain geometry, hybridisation and magnetic property of [CoF6]−3 (Atomic number of Co = 27).
View Solution
