Out of but-1-yne or but-1-ene which has higher dipole moment?
Video Solution
Text Solution
H5C2−C≡C−HBut-1-yne 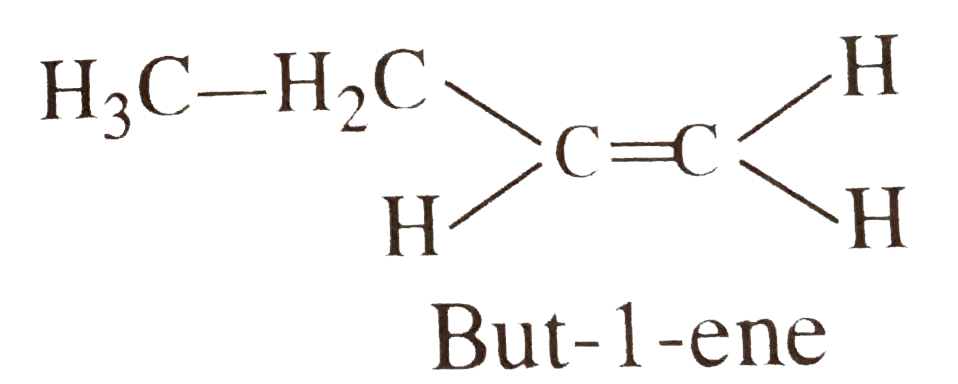
Buy-1-tne has more dipole moment than but -1-ene because, ≡C−H bond is more polar than = C-H bond Actually, the electronegativity of sp-C in alkyne is more than that of sp2−C in alkene.
|
Similar Questions
Out of NH3 and NF3 which has a higher Dipole moment?
View SolutionWhich out of NH3 and NF3 has higher dipole moment and why?
View SolutionWhich out of NH3 and NF3 has higher dipole moment and why ?
View SolutionWhich out of NH3 and NF3 has higher dipole moment and why?
View SolutionOut of CS2 and OCS which have higher dipole moment and why?
View SolutionOut of but-1-yne or but-1-ene which has higher dipole moment?
View SolutionThe IUPAC name of the compound CH3-CH=CH-C -= CH is Pent-2-en-4-yne Pent-1-en-4yne Pent-3-en-1-yne Pent-2-en-5-yne
View SolutionDraw structure of following IUPAC names. (i)Hexa- 2,4 -diyne , (ii)Pent- 3 -ene- 1 -yne (iii)Pent- 1 -en- 4 -yne , (iv)Pent- 1 -en- 3 -yne
View Solution

