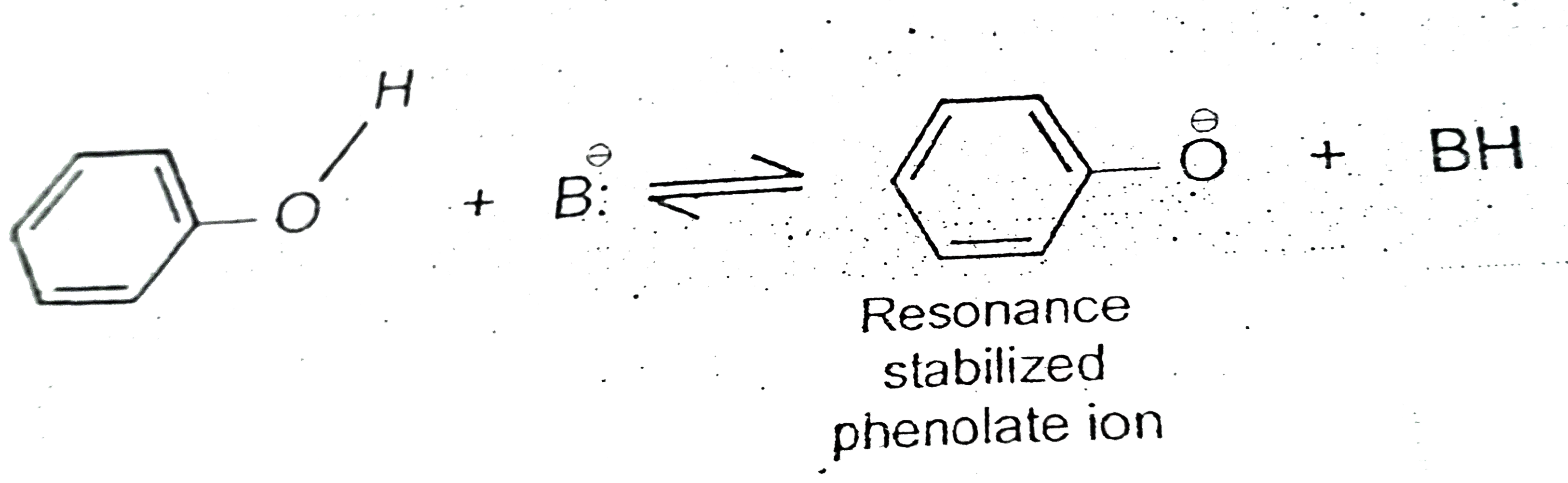
Phenols are more acidic than aliphatic alcohols acidity of phenols can be further increased by the introduction of electron withdrawing groups in aromatic ring .Acidic nature of phenol is because of the resonance stabilization of phenoxide ion
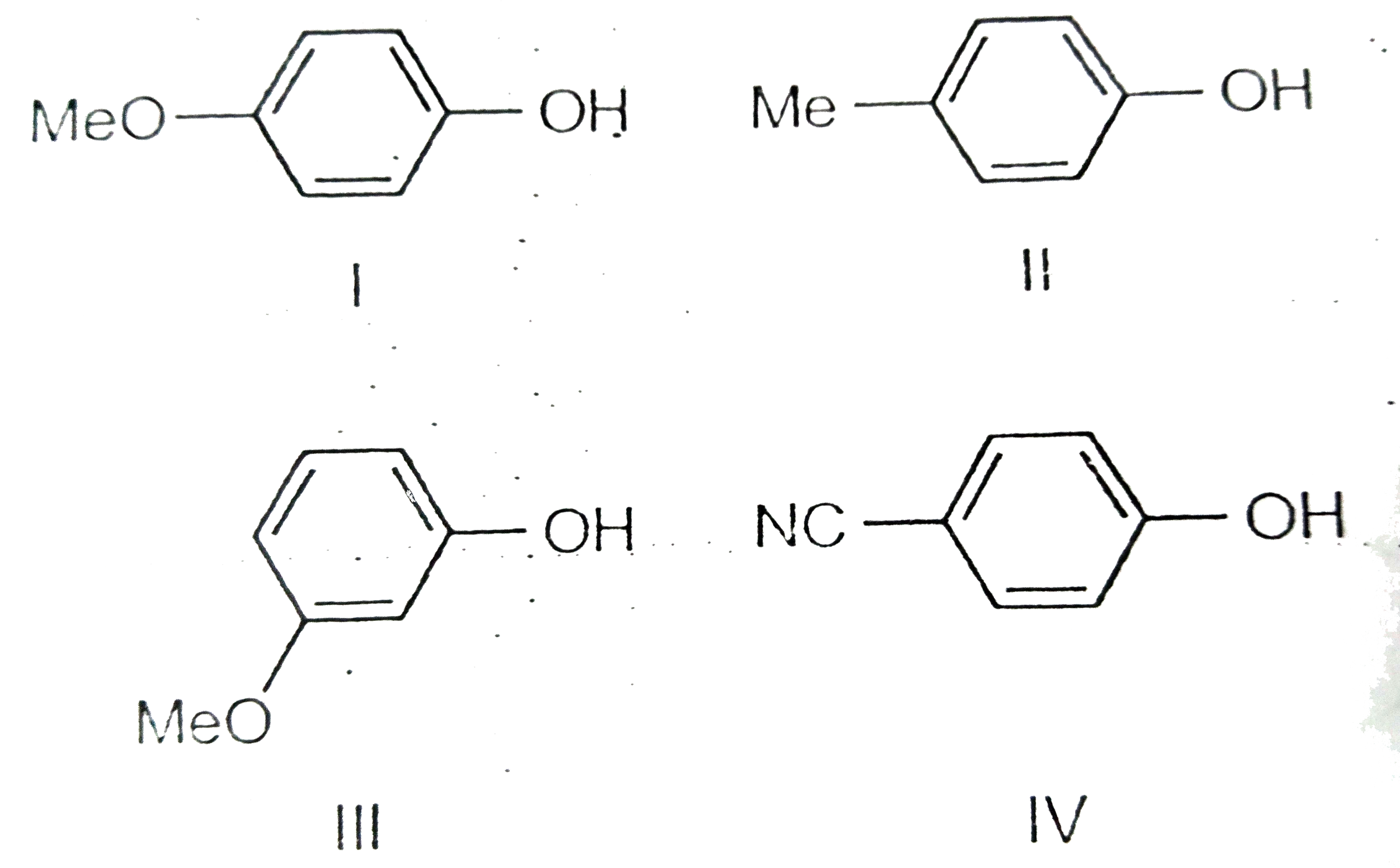
Arrange the given phanols in the increasing order of acidic strength
A
I<II<III<IV
B
II<I<III<IV
C
I<IV<III<II
D
II<IV<III
Video Solution
Text Solution
The correct Answer is:B
NA
|
Similar Questions
Phenols are more acidic than aliphatic alcohols acidity of phenols can be further increased by the introduction of electron withdrawing groups in aromatic ring .Acidic nature of phenol is because of the resonance stabilization of phenoxide ion
Which of the following will evolve CO2 gas with aqueous NaHCO3?
View SolutionPhenol is more acidic than ethanol.
View Solution
Knowledge Check
Phenols are more acidic than aliphatic alcohols because
Aphenoxide ion is stabilised by resonanceBphenols are more soluble in polar solventsCphenoxide ion do not have resonanceDalcohols do not loose H-atom at allAcidic nature of phenol is due to
Aphenolic groupBbenzene groupChydrogen bondingDresonance stabilization of phenoxide ionAcidic nature of phenol is due to
Aphenolic groupBbenzene groupChydrogen bondingDresonance stabilisation of phenoxide ion

