The ionisation potentials of Li and K are 5.4 and 4.3 eV respectively. The ionization potential of Na will be:
9.7 eV
1.1 eV
4.9 eV
cannot be calculated
The correct Answer is:C
Step by step video, text & image solution for The ionisation potentials of Li and K are 5.4 and 4.3 eV respectively. The ionization potential of Na will be: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- PERIODIC PROPERTIESBOOK - VK JAISWALCHAPTER - PERIODIC PROPERTIESEXERCISE - Level 2
102 Videos
- PERIODIC PROPERTIESBOOK - VK JAISWALCHAPTER - PERIODIC PROPERTIESEXERCISE - MATCH THE COLUMN
13 Videos
- PERIODIC PROPERTIESBOOK - VK JAISWALCHAPTER - PERIODIC PROPERTIESEXERCISE - SUBJECTIVE PROBLEMS
22 Videos
- p-BLOCK ELEMENTSBOOK - VK JAISWALCHAPTER - p-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
36 Videos
- QUALITATIVE INORGANIC ANALYSISBOOK - VK JAISWALCHAPTER - QUALITATIVE INORGANIC ANALYSISEXERCISE - SUBJECTIVE PROBLEMS
5 Videos
Similar Questions
A compound contains three elements A,B and C, if the oxidation number of A=+2B=+5 and C=−2 then possible formula of the compound is
View Solution
View SolutionThe number of electrons for Zn2+ cation that have the value of azimuthal quantum number =0 is:
View SolutionIn phosphorus acid, If X is number of non-bonding electron pairs. Y is number of σ-bonds and Z is number of π-bonds. Then calculate value of "YxZ-X".
View SolutionConsider the following reaction
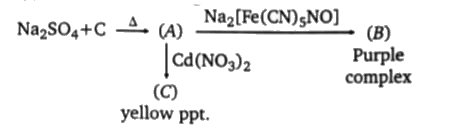
Then calculate value of ∣∣X2−Y2∣∣ (where X and Y are total number of electrons present t_(2g) and e_(g) orbitals respectively in d-block metal ion of compound B).
View SolutionThe first ionisation potentials (eV) of Be and B respectively are
View SolutionIf the IP of Na is 5.48 eV, the ionsation potential of K will be
View SolutionThe ionisation potentials of Li and K are 5.4 and 4.3 eV respectively. The ionization potential of Na will be:
View SolutionIf the IP of Na is 5.48 eV, the ionsation potential of K will be
View SolutionThe first ionisation potentials (eV) of Be and B respectively are
View SolutionThe ionisation potential of H-atom is 13.6 eV. Calculate the ionisation potential of He+ and Li2+ ions.
View SolutionThe first ionization potential (eV) of N and P respectively are
View SolutionNa का प्रथम आयनन विभव 5.1 eV है। Na+ की इलैक्ट्रॉन प्राप्ति एन्थैल्पी निम्न होगी :
View Solutionयदि Na का आयनन विभव 5.48ev हो तो K का आयनन विभव होगा
View SolutionArrange the following
Li, K, Na, H [In the decreasing order of their ionization potential].
View Solution

