Which of the following statement is correct regarding p-V graph?
1 slop of p-V graph in an isothermal process is pv
2 slope of p-V graph in an adiabatic process is −pv
3 slope of p-V graph in an isochoric process is γpv
4 slope of p-V graph in an isobaric porcess is zero Solution in Telugu
1,3,4 are correct
2,3 are correct
1,4 are correct
2,3,4 are correct
The correct Answer is:C
(i) and (IV) options are correct
(i) Slope of p-v graph in an siothermal process is −pV this is correct.

for isothemal process
slope of p-v curve dpdv−pv
(ii) Slope of p-v group in an adiabatic process is −pv which is wrong

(iii) Slope of p-v graph in an isochoric process is −γpv this option is wrong

( d ) Slope of p-v graph in an isobaric process is zero .this option is correct

p-v curve is a striaght line parallel to volume axis slope of p-v curve for an isobaric process dpdv=0
Similar Questions
Plot P-V, V-T and p-T graph correspongidng to the P-T graph for an ideal gas shown in figure.
View SolutionThe slope of P-V graph for isochoric process is
View SolutionThe slope of adiabatic P-V curve is_____times the slope of isothermal P-V curve in the process.
View SolutionAn ideal gas follows a process PVγ = constant where γ = adiabatic exponent.The slope of P-V graph will be represented by
View SolutionThe slope of P-V graph for isochoric process is
View Solutionp−T curve for a cyclic process is as shown
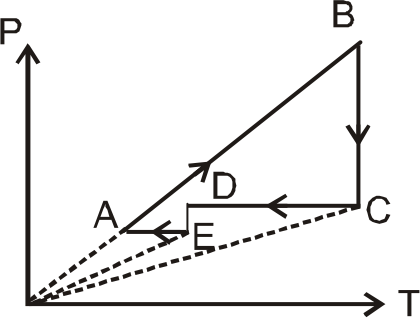
P−V graph for this process will be:
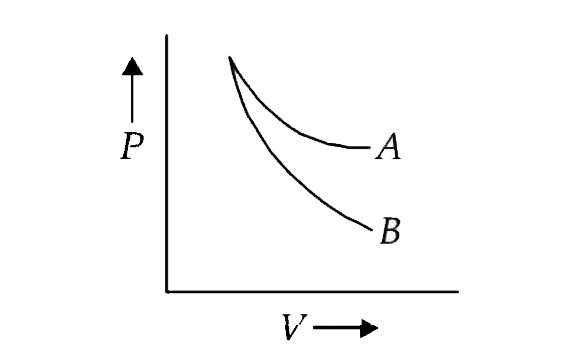
View SolutionThe curves A and B in the figure shown P-V graphs for an isothermal and an adiabatic process for an idea gas. The isothermal process is represented by the curve A.
View SolutionAssertion: P v/s 1V graph is straight line for adiabatic process.
Reason: PV=constant for adiabatic process.
View Solution

