The slope of adiabatic P-V curve is_____times the slope of isothermal P-V curve in the process.Solution in Odia
Step by step video & image solution for The slope of adiabatic P-V curve is_____times the slope of isothermal P-V curve in the process. by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
A glass window pane 1 m by 1 m is 25 mm thick. If the show outside maintains the outer surface at 0∘C and the heat of the room maintains the inner surface at 6∘C ,Thermal conductivity of glass in S.I unit is 0.2, how much heat will be conducted through the glass in 1 day ?
View SolutionFind out the magnitude of the orthogonal components of a displacement vector in space .
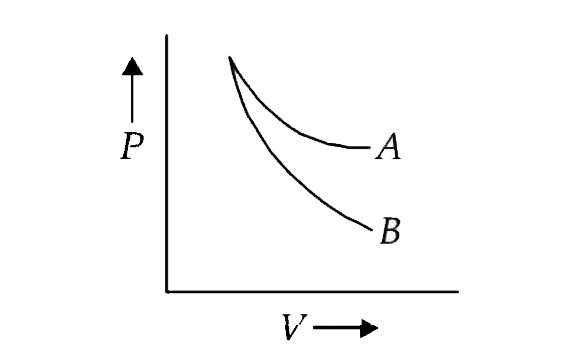
View SolutionThe curves A and B in the figure shown P-V graphs for an isothermal and an adiabatic process for an idea gas. The isothermal process is represented by the curve A.
View SolutionAssertion: Isothermal and adiabatic, two processes are shown on p-V diagram. Process-1 is aidabatic and process-2 is isothermal. Reason: At a given point, slope of adiabatic process =gammaxx slope of isothermal process.
View SolutionThe ratio of slopes of P−V plots for reversible adiabatic process and reversible isothermal process of an ideal gas is equal to :
View SolutionThe ratio of slopes of adiabatic and isothermal curves is
View SolutionFor a p-V plot, the slope of an adiabatic curve = x × slope of isothermal at the same point. Here x is :
View SolutionThe slope of adiabatic curve is _________________than the slope of an isothermal curve.
View SolutionThe slope of isothermal and adiabatic curves are related as
View SolutionDraw P-V curves for isothermal and adiabatic processes of an ideal gas.
View SolutionThe relation between the slope of isothermal curve and slope of adiabatic curve is
View SolutionShow that the slope of p−V diagram is greater for an adiabatic process as compared to an isothermal process.
View SolutionWhy is p-V curve for adiabatic process steeper than that for isothermal process?
View SolutionAssertion: Isothermal and adiabatic, two processes are shown on p-V diagram. Process-1 is aidabatic and process-2 is isothermal. Reason: At a given point, slope of adiabatic process =gammaxx slope of isothermal process.
View SolutionThe curves A and B in the figure shown P-V graphs for an isothermal and an adiabatic process for an idea gas. The isothermal process is represented by the curve A.
View Solution

