The first ionisation energy (IE1) and second ionisation energy (IE2) of elements X, Y and Z are given below.

Which one of the above elements is the most reactive metal, the least reactive metal and a noble gas? Solution in Tamil
Noble gases: Ioniation energy ranging from 2372KJmol−1 to 1037kJmol−1. For element X, the IE1 value is in the range of noble gas, moreover for this element both IE1 and IE2 are higher and hence X is the noble gas. For Y, the first ionisation energy is low and second ionisation energy is very high and hence Y is most reactive metal.
For Z, both IE1 and IE2 are higher and hence it is least reactive.
Topper's Solved these Questions
- PERIODIC CLASSIFICATION OF ELEMENTS BOOK - FULL MARKSCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTS EXERCISE - Additional Questions(Match the follwing)
5 Videos
- PHYSICAL AND CHEMICAL EQUILIBRIUMBOOK - FULL MARKSCHAPTER - PHYSICAL AND CHEMICAL EQUILIBRIUMEXERCISE - ADDITIONAL QUESTIONS SOLVED (NUMERICAL PROBLEMS)
3 Videos
Similar Questions
Match the following columns
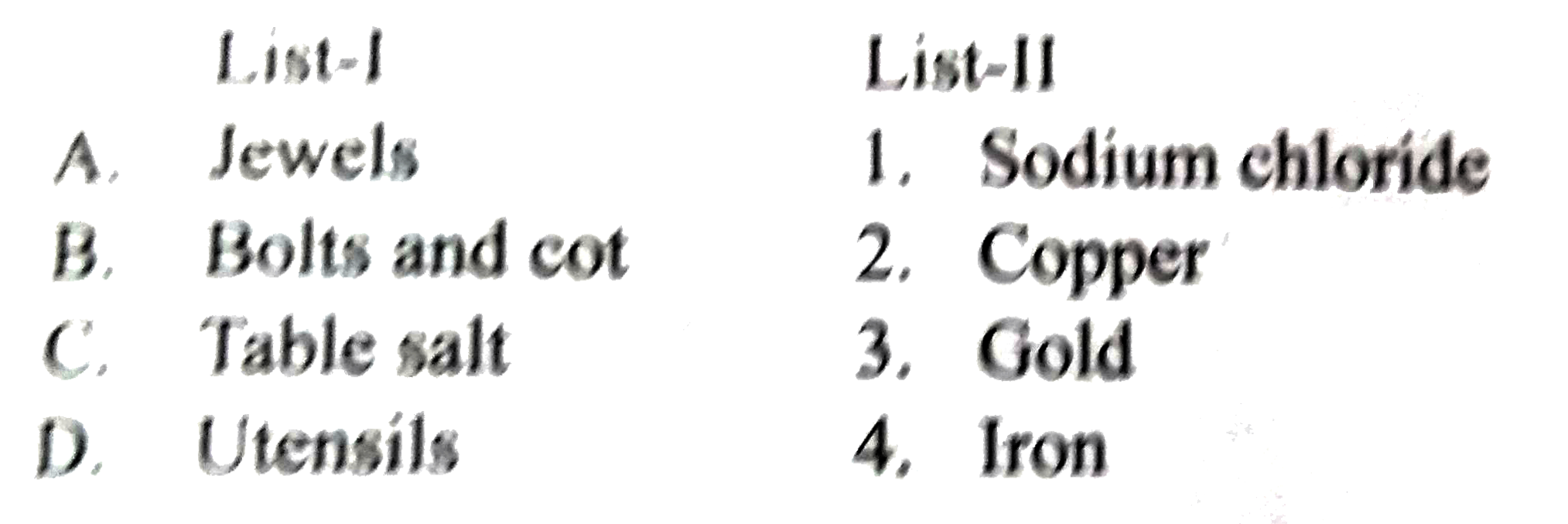
View SolutionFind the value of Kc for each of the following equilibria from the value of Kp
(a) 2NOCl(g)⇔2NO(g)+Cl2(g),KP=1.8×10−2 atm at 500 K
(b) CaCO3(s)⇔CaO(s)+CO2(g),KP=167 atm at 1073 K
View SolutionThe first and second ionization enthalpies and electron gain enthalpy of some elements are as given below: Which of these elements is the most reactive metal?
View SolutionThe first (IE_1) and the second (IE_2) ionisation enthalpies (kJ mol^(-1)) of three elements are given below: {:(,I,II,III),(IE_1,403,549,1142),(IE_2,2640,1060,2080):} Identify the element which is likely to be:- a non metal
View SolutionThe first (IE_1) and the second (IE_2) ionisation enthalpies (kJ mol^(-1)) of three elements are given below: {:(,I,II,III),(IE_1,403,549,1142),(IE_2,2640,1060,2080):} Identify the element which is likely to be:- an alkali metal
View SolutionThe first (IE_1) and the second (IE_2) ionisation enthalpies (kJ mol^(-1)) of three elements are given below: {:(,I,II,III),(IE_1,403,549,1142),(IE_2,2640,1060,2080):} Identify the element which is likely to be:- an alkaline earth metal
View SolutionThe first (IE_1) and second (IE_2) ionisation enthalpies ("kJ mol"^(-1)) of a few elements designated by Roman numerals are shown below. {:(,,IE_(1),IF_(2)),(,I,2372,5251),(,III,900,1760):} {:(,,IE_(1),IE_(2)),(,II,520,7300),(,IV,1680,3380):} Which of the above element is likely to be: a reactive metal
View SolutionThe first (IE1) and second (IE2) ionisation enthalpies (kJ mol−1) of a few elements designated by Roman numerals are shown below.
IE1IF2I23725251III9001760 IE1IE2II5207300IV16803380
Which of the above element is likely to be:
a reactive non-metal
View SolutionThe first (IE1) and second (IE2) ionisation enthalpies (kJ mol−1) of a few elements designated by Roman numerals are shown below.
IE1IF2I23725251III9001760 IE1IE2II5207300IV16803380
Which of the above element is likely to be:
a reactive metal
View SolutionThe first (IE1) and second (IE2) ionisation enthalpies (kJ mol−1) of a few elements designated by Roman numerals are shown below.
IE1IF2I23725251III9001760 IE1IE2II5207300IV16803380
Which of the above element is likely to be:
a reactive non-metal
View SolutionThe first ionisation energy (IE1) and second ionisation energy I(E2) of elements X,Y and Z are given below.
ElementIE1(kJ mol−1)IE2(kJmol−1)X23705250Y5227298Z16803381
Which one of the above elements is the most reactive metal, the least reactive metal and a noble gas ?
View SolutionThe most reactive noble gas element is
View SolutionThe first (IP1) and the second (IP2) ionisation potential (kJ mol 1) of a few elements designed by roman numerals are shown below
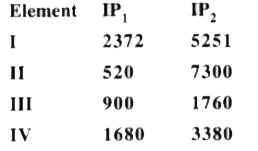
Identify the element which are likely to be a noble gas and a reactive metal respectively
View SolutionThe first (DletaH) and second ionisation enthalipies (DeltaH_(2)) in kJmol^(-1) and the electron gain enthalpy (DeltaHg) in kJmol^(-1) of a few elements are given below: Which element is likely a) the least reactive element c) the most reactive non-metal b) the most reactive metal d) the least reactive non-metal f) the metal which can form a predominantly stable covalent halide of the formula MX.
View SolutionThe first (IE1) and the second (IE2) ionisation energies (kJ mol−1) of a few elements are given below :
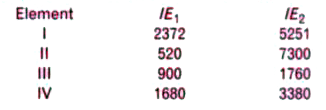
Which of the above elements is likely to be
a reactive metal?
View Solution

