Why the bond angle in solid phase of H2O2is reduced when compared to as phase of H2O2?Solution in Tamil
Video Solution
Text Solution
Both the gas phase and solid phase, H2O2 adopt a skew configuration due to the repulsive interaction of the OH bonds with lone pairs of electrons on each oxygen atoms. Indeed, it is the smallest molecule known to show hindered rotation about a single bond. In the solid phase, the dihedral angle is sensitive and hydrogen bonding decreases from 111.5° in the gas phase to 90.2∘ in the solid-phase.
|
Similar Questions
Discuss the structure of H2O2 molecule.Explain why the shape of H2O2 molecule in the solid phase differs from that in the gas phase.
View SolutionThe dihedral angle of H_(2)O_(2) in solid phase is
View SolutionThe angle between the planes of H_(2)O_(2) molecule in gaseous phase is
View Solution(i) Draw the gas phase and solid phase structure of H2O2.
(ii) H2O2 is a better oxidising agnet than water . Explain .
View SolutionWhich of the following is a true structure of H_(2)O_(2) in solid phase?
View SolutionTwo structures of H2O2 are drawn below. Identify the phases X and Y of H2O2.
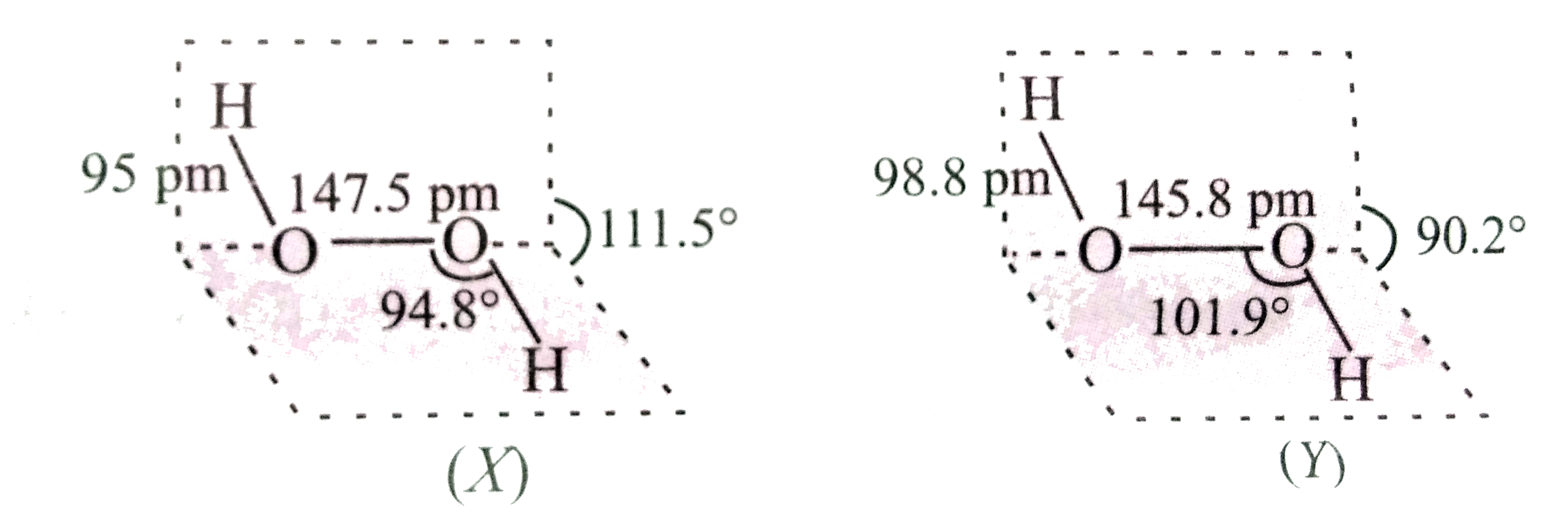
View SolutionAssertion : In gaseous phase, H2O and H2O2 both have bent structures.
Reason Bond angle of both H2O and H2O2 is 104.5∘.
View Solution
