Which of the following compound is paramagnetic ?


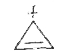
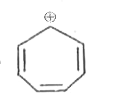
The correct Answer is:1
To determine which of the given compounds is paramagnetic, we need to understand the concepts of paramagnetism and diamagnetism.
Step 1: Understand Paramagnetism and Diamagnetism
- Paramagnetic substances are those that have unpaired electrons. These substances are attracted to an external magnetic field.
- Diamagnetic substances have all their electrons paired and are repelled by an external magnetic field.
Step 2: Identify Unpaired Electrons
To identify if a compound is paramagnetic, we need to look for unpaired electrons in its electronic configuration.
Step 3: Analyze the Compounds
1. Determine the electronic configuration of each compound given in the options.
2. Count the number of unpaired electrons in each compound.
Step 4: Apply Molecular Orbital Theory (MOT)
- For compounds with π electrons, we can use Molecular Orbital Theory (MOT) to determine the arrangement of electrons in molecular orbitals.
- In MOT, if there are unpaired electrons in the molecular orbitals, the compound will be paramagnetic.
Step 5: Conclusion
After analyzing the compounds based on the above steps, we can conclude which compound is paramagnetic.
Example Analysis
Let's say we have the following options:
1. Compound A
2. Compound B
3. Compound C
4. Compound D
- If Compound A has 2 unpaired electrons, Compound B has 0 unpaired electrons, Compound C has 1 unpaired electron, and Compound D has 3 unpaired electrons, then:
- Compound A (2 unpaired) - Paramagnetic
- Compound B (0 unpaired) - Diamagnetic
- Compound C (1 unpaired) - Paramagnetic
- Compound D (3 unpaired) - Paramagnetic
In this case, all compounds with unpaired electrons (A, C, D) are paramagnetic.
Final Answer
Based on the analysis, the correct answer is the compound with unpaired electrons, which is paramagnetic.
---
- Paramagnetic substances are those that have unpaired electrons. These substances are attracted to an external magnetic field.
- Diamagnetic substances have all their electrons paired and are repelled by an external magnetic field.
To identify if a compound is paramagnetic, we need to look for unpaired electrons in its electronic configuration.
1. Determine the electronic configuration of each compound given in the options.
2. Count the number of unpaired electrons in each compound.
- For compounds with π electrons, we can use Molecular Orbital Theory (MOT) to determine the arrangement of electrons in molecular orbitals.
- In MOT, if there are unpaired electrons in the molecular orbitals, the compound will be paramagnetic.
After analyzing the compounds based on the above steps, we can conclude which compound is paramagnetic.
Let's say we have the following options:
1. Compound A
2. Compound B
3. Compound C
4. Compound D
- Compound A (2 unpaired) - Paramagnetic
- Compound B (0 unpaired) - Diamagnetic
- Compound C (1 unpaired) - Paramagnetic
- Compound D (3 unpaired) - Paramagnetic
Based on the analysis, the correct answer is the compound with unpaired electrons, which is paramagnetic.
Topper's Solved these Questions
- HYDROCARBONSBook:AAKASH INSTITUTEChapter:HYDROCARBONSExercise:Assignment(Section - C) (Previous Years Questions)
Explore 69 Videos
- HYDROCARBONSBook:AAKASH INSTITUTEChapter:HYDROCARBONSExercise:Assignment(Section - D) (Assertion -Reason Type Question)
Explore 24 Videos
- HYDROCARBONSBook:AAKASH INSTITUTEChapter:HYDROCARBONSExercise:Assignment(Section - A) (Obejctive type question)
Explore 55 Videos
- HALOALKANES AND HALOARENES Book:AAKASH INSTITUTEChapter:HALOALKANES AND HALOARENES Exercise:ASSIGNMENT SECTION -D
Explore 15 Videos
- HYDROGENBook:AAKASH INSTITUTEChapter:HYDROGENExercise:ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)
Explore 15 Videos
Similar Questions
Knowledge Check
Which of the following compounds is paramagnetic ?
A[Ni(CO)4]BK2[Ni(CN)4]CK3[CoF6]D[Cr(NH3)6]Cl3Which of the following comnpounds is paramagnetic?
ACOBNOCO2−2DO3Which of the following compound gives paramagnetic gas on heating :-
ALiNO3BNaNO3CKNO3DAll of these
Which of the following compounds are paramagnetic in nature?
View SolutionWhich of the following compounds are paramagnetic in nature ?
View SolutionWhich of the following compounds give paramagnetic gas on decomposition
(i) Pb(NO3)2
(ii) LiNO3
(iii) NaNO3
(iv) NH4NO2
View SolutionWhich among the following compounds is paramagnetic ?
View SolutionWhich of the following complexes is paramagnetic
View Solution
