StateP1,V1,T1ARev−−→State BP2,V2,T2: If P1=P2 thenSolution in Telugu
ΔSuniverse=0
ΔSuniverse=1
ΔSuniverse>0
ΔSuniverse<0
Step by step video & image solution for underset(P_(1), V_(1), T_(1))("State") A overset("Rev")rarr underset(P_(2), V_(2), T_(2))("State B"): If P_(1) = P_(2) then by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
A system Xundergoes following changes
XP1V1T1→WP2V2T1→ZP3V2T2→XP1V1T1
The overall process may be called as
View SolutionA sample of gas changes from p1,V1 and p2. V2andT2 by one path and then back to p1,V1andT1. ΔU for the process is :
View Solutionunderset(P_(1), V_(1), T_(1))("State") A overset("Rev")rarr underset(P_(2), V_(2), T_(2))("State B") : If P_(1) = P_(2) then
View SolutionA gas with CP/CV= goes from an initial state (P1,V1,T1) to a final state (P2,V2,T2) through an adiabatic process. The work done by the gas is
a)nR(T1−T2)γ−1
b)P1V1−P2V2γ−1
c) nRγ(T1−T2)
View SolutionA gas with C_(P)//C_(V)=gamma goes from an initial state (P_(1), V_(1), T_(1)) to a final state (P_(2), V_(2), T_(2)) through an adiabatic process. The work done by the gas is a) (nR(T_(1)-T_(2)))/(gamma-1) b) (P_(1)V_(1)-P_(2)v_(2))/(gamma-1) c) nR gamma(T_(1)-T_(2))
View SolutionFor an ideal gas, an illustratio of three different paths A(B+C) and (D+E) from an initial state P_(1), V_(1), T_(1) to a final state P_(2), V_(2),T_(1) is shown in the given figure. Path A represents a reversible isothermal expansion form P_(1),V_(1) to P_(2),V_(2) , Path (B+C) represents a reversible adiabatic expansion (B) from P_(1),V_(1),T_(1)to P_(3),V_(2),T_(2) followed by reversible heating the gas at constant volume (C) from P_(3),V_(2),T_(2) to P_(2),V_(2),T_(1) . Path (D+E) represents a reversible expansion at constant pressure P_(1)(D) from P_(1),V_(1),T_(1) to P_(1),V_(2),T_(3) followed by a reversible cooling at constant volume V_(2)(E) from P_(1),V_(2),T_(3) to P_(2),V_(2),T_(1) . What is q_(rev) , for path (A) ?
View SolutionFor an ideal gas, an illustration of three different paths A,(B+C) and (D+E) from an initial state P1,V1,T1 to a final state P2,V2,T1 is shown in the given figure.
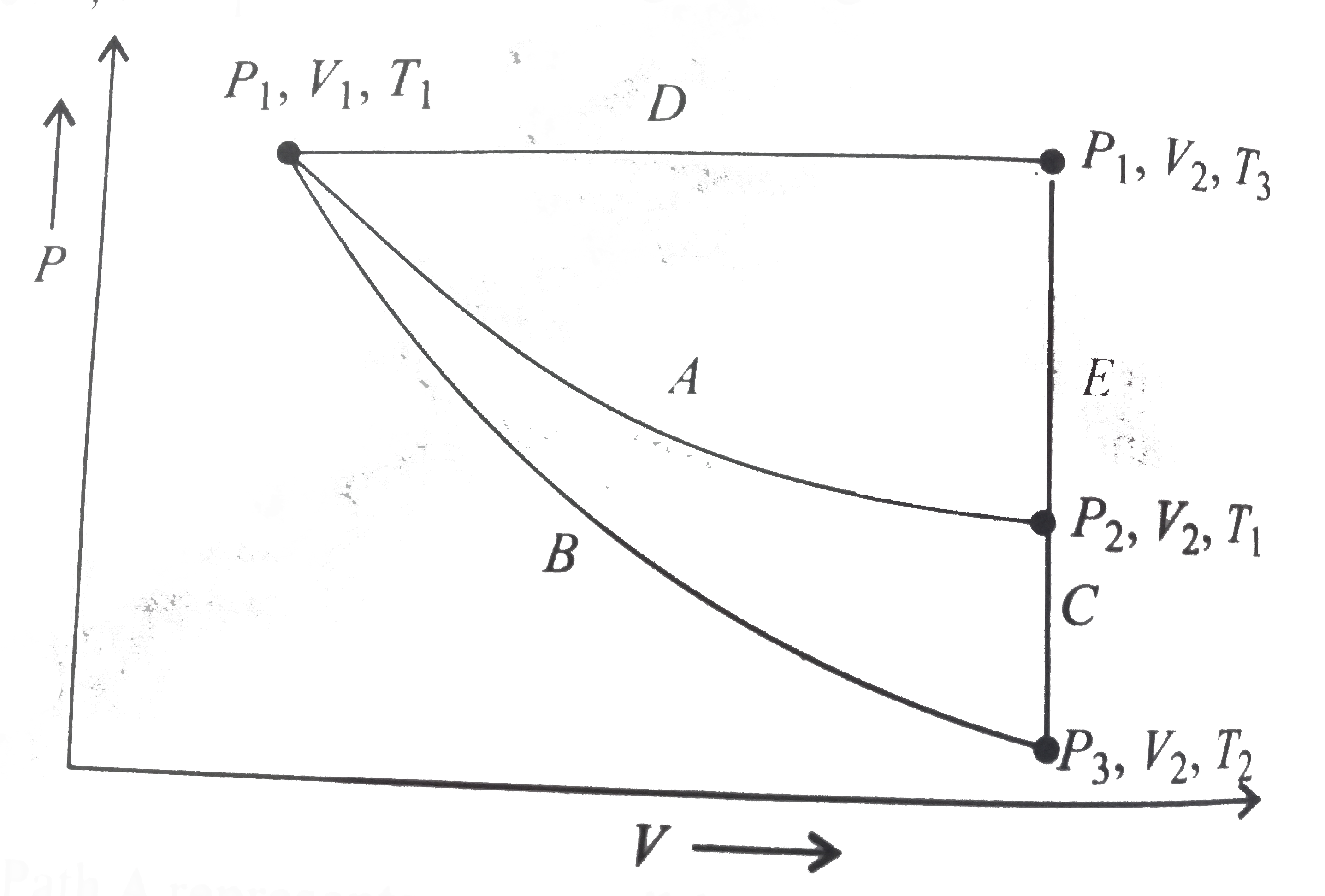
Path Arepresents a reversible isothermal expansion form P1,V1 to P2,V2, Path (B+C) represents a reversible adiabatic expansion (B) from P1,V1,T1→P3,V2,T2 followed by reversible heating the gas at constant volume (C)from P3,V2,T2 to P2,V2,T1. Path (D+E) represents a reversible expansion at constant pressure P1(D) from P1,V1,T1 to P1,V2,T3 followed by a reversible cooling at constant volume V2(E) from P1,V2,T3→P2,V2,T1.
What is qrev, for path A?
View SolutionFor an ideal gas, an illustratio of three different paths A(B+C) and (D+E) from an initial state P_(1), V_(1), T_(1) to a final state P_(2), V_(2),T_(1) is shown in the given figure. Path A represents a reversible isothermal expansion form P_(1),V_(1) to P_(2),V_(2) , Path (B+C) represents a reversible adiabatic expansion (B) from P_(1),V_(1),T_(1)to P_(3),V_(2),T_(2) followed by reversible heating the gas at constant volume (C) from P_(3),V_(2),T_(2) to P_(2),V_(2),T_(1) . Path (D+E) represents a reversible expansion at constant pressure P_(1)(D) from P_(1),V_(1),T_(1) to P_(1),V_(2),T_(3) followed by a reversible cooling at constant volume V_(2)(E) from P_(1),V_(2),T_(3) to P_(2),V_(2),T_(1) . What is q_(rev) , for path (D+E) ?
View Solution
