Describe the construction and working of standard hydrogen electrode. Solution in Kannada
Video Solution
Text Solution
Construction: The standard hydrogen electrode consists of a platinum wire soldered to a glass tube. It is attached to a platinum foil coated with platinum black. Platinum wire is to provide connections to other electrode systems and platinum black is for adsorption of hydrogen gas. The glass tube is for adsorption by gas jacket having an inlet at the top for H2gas and holes at the bottom for its escape. The whole apparatus is dipped in 1M HCl.
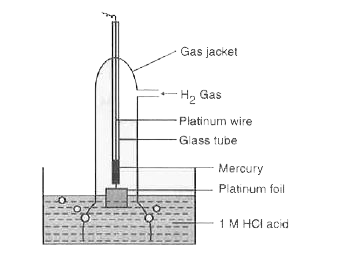
Working: H2 gas at 1atm is let into the gas jacket. This increases the pressure inside and acid level is pused down. Platinum black adsorbs the H2 gas and remaining gas escape through the holes pressure falls again and acid level is raised. Adsorbed hydrogen comes in contact with hydrogen ions of solution. There exists an equilibrium between the ions & hydrogen.
H2↔2H++2e−
THe equilibrium is responsible for the potential of hydrogen electrode. IT is used to measure the potential of other electrode systems. It is symbollically represented as Pt,H2(1atm)∣H+(1M).
|
Similar Questions
Describe the constitution of standard hydrogen electrode.
View SolutionDescribe the construction and working of standard hydrogen electrode.
View SolutionDescribe the construction of standard hydrogen electrode.
View SolutionDescribe the working of standard hydrogen electrode.
View SolutionWhat is the standard hydrogen electrode? Briefly describe the measurement of potential of a single electron.
View SolutionDescribe the construction and working of standard hydrogen electrode.
View SolutionExpress standard Hydrogen electrode.
View SolutionElectrode potential || EMF OF Cell || Standard Hydrogen Electrode || Measurement OF Standard Electrode Potential || Significance OF Standard Electrode Potential
View Solution
