How is 1 - propoxypropane synthesised from propan -1 - ol? Write mechanism of this reaction?
Text Solution
(a) 1 - propoxypropane can be synthesised using Williamson synthesis as follows :
(i) 3CH3CH2CH2OHPropan - 1 - ol+PBr3→3CH3CH2CH2Br+H3PO31 - Bromopropane
(ii) 2CH3CH2CH2OHPropan - 1 - ol+2Na→2CH3CH2CH2O−Na+Sodium propoxide+H2
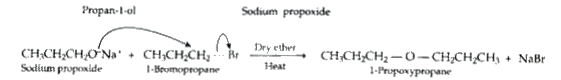
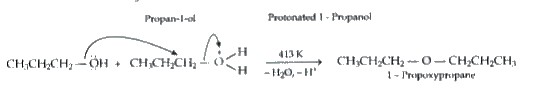
(b) It can be also be synthesised by dehydration of 1 - propanol with conc. H2SO4 at 413 K.
|
Topper's Solved these Questions
- ALCOHOLS, PHENOLS AND ETHERSBook:U-LIKE SERIESChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS
Explore 14 Videos
- ALCOHOLS, PHENOLS AND ETHERSBook:U-LIKE SERIESChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:MULTIPLE CHOICE QUESTIONS
Explore 19 Videos
- ALCOHOLS, PHENOLS AND ETHERSBook:U-LIKE SERIESChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:SELF ASSESSMENT TEST (SECTION A)(MULTIPLE CHOICE QUESTIONS)(CHOOSE THE CORRECT OPTION)
Explore 6 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:U-LIKE SERIESChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:SELF ASSESSMENT TEST (SECTION B)
Explore 1 Video
Similar Questions
Knowledge Check
Propan-1-ol can be prepared from propene by
AH2O/H2SO4BHg(OAc)2/H2O followed by NaBH4CB2H6 followed by H2ODCH3CO2H/H2SO42-methyl propan-1-ol is obtained from
APhenol + conc.H2SO4BPhenol + conc. HNO3Cethanal+ (CH3)+2CHMgXDmethanal + (CH3)2CHMgX
How will you convert propene to propan -1-ol ?
View SolutionHow is propan - 2 - ol prepared by hydration of an alkene?
View SolutionHow will you convert propanone to propan-2-ol?
View SolutionHow will you convert propanone to propan-2-ol?
View SolutionHow is propan-1-0l prepared from (1) propanal (2) propanoic acid?
View SolutionHow will you convert?
Propane 2 ol to 1-Bromo propane
View Solution

