Redox is a reaction in which both oxidation and reduction will take place simultaneously . It is obvious that if one substance gives electron there must be another substance to accept these electrons . In some reactions, same substance is reduced as well as oxidised, these reactions are termed as disproportionation reactions.
For calculating equivalent mass in redox reaction change in oxidtaion number is realted to n-factor which is reciprocal of molar ratio.
How many moles of KMnO4 are reacted with one mole of ferrous oxalate in acidic medium ?
25
15
35
53
The correct Answer is:C
To solve the problem of how many moles of KMnO4 react with one mole of ferrous oxalate (FeC2O4) in acidic medium, we will follow these steps:
Step 1: Identify the oxidation states
- In ferrous oxalate (FeC2O4), iron (Fe) is in the +2 oxidation state (Fe²⁺).
- In KMnO4, manganese (Mn) is in the +7 oxidation state (MnO4⁻).
Step 2: Write the half-reactions
1. Oxidation half-reaction:
- Fe²⁺ → Fe³⁺ + e⁻ (loses 1 electron)
- The oxalate ion (C2O4²⁻) is oxidized to CO2:
- C2O4²⁻ → 2 CO2 + 2 e⁻ (loses 2 electrons)
Overall oxidation reaction:
Fe2++C2O2−4→Fe3++2CO2+3e−
2. Reduction half-reaction:
- MnO4⁻ + 8 H⁺ + 5 e⁻ → Mn²⁺ + 4 H2O (gains 5 electrons)
Step 3: Balance the electrons
To balance the overall reaction, we need to ensure that the number of electrons lost in oxidation equals the number of electrons gained in reduction.
- From the oxidation half-reaction, we have a total of 3 electrons lost (from Fe²⁺ and C2O4²⁻).
- From the reduction half-reaction, we have 5 electrons gained.
To balance the electrons, we can multiply the oxidation half-reaction by 5 and the reduction half-reaction by 3.
Step 4: Write the balanced equation
1. Multiply the oxidation half-reaction by 5:
5Fe2++5C2O2−4→5Fe3++10CO2+15e−
2. Multiply the reduction half-reaction by 3:
3MnO−4+24H++15e−→3Mn2++12H2O
Step 5: Combine the half-reactions
Now, we can combine both half-reactions:
5Fe2++5C2O2−4+3MnO−4+24H+→5Fe3++10CO2+3Mn2++12H2O
Step 6: Determine the mole ratio
From the balanced equation, we see that:
- 5 moles of ferrous oxalate react with 3 moles of KMnO4.
To find out how many moles of KMnO4 react with 1 mole of ferrous oxalate:
If 5 moles of FeC2O4 require 3 moles of KMnO4, then 1 mole of FeC2O4 requires 35 moles of KMnO4.
Final Answer
Thus, 1 mole of ferrous oxalate reacts with 0.6 moles (or 3/5 moles) of KMnO4 in acidic medium.
---
- In ferrous oxalate (FeC2O4), iron (Fe) is in the +2 oxidation state (Fe²⁺).
- In KMnO4, manganese (Mn) is in the +7 oxidation state (MnO4⁻).
1. Oxidation half-reaction:
- Fe²⁺ → Fe³⁺ + e⁻ (loses 1 electron)
- The oxalate ion (C2O4²⁻) is oxidized to CO2:
- C2O4²⁻ → 2 CO2 + 2 e⁻ (loses 2 electrons)
Fe2++C2O2−4→Fe3++2CO2+3e−
- MnO4⁻ + 8 H⁺ + 5 e⁻ → Mn²⁺ + 4 H2O (gains 5 electrons)
To balance the overall reaction, we need to ensure that the number of electrons lost in oxidation equals the number of electrons gained in reduction.
- From the reduction half-reaction, we have 5 electrons gained.
1. Multiply the oxidation half-reaction by 5:
5Fe2++5C2O2−4→5Fe3++10CO2+15e−
3MnO−4+24H++15e−→3Mn2++12H2O
Now, we can combine both half-reactions:
5Fe2++5C2O2−4+3MnO−4+24H+→5Fe3++10CO2+3Mn2++12H2O
From the balanced equation, we see that:
- 5 moles of ferrous oxalate react with 3 moles of KMnO4.
If 5 moles of FeC2O4 require 3 moles of KMnO4, then 1 mole of FeC2O4 requires 35 moles of KMnO4.
Thus, 1 mole of ferrous oxalate reacts with 0.6 moles (or 3/5 moles) of KMnO4 in acidic medium.
Topper's Solved these Questions
- REDOX REACTIONSBook:AAKASH INSTITUTE ENGLISHChapter:REDOX REACTIONSExercise:Assignment (Section E) (Assertion-Reason Type Questions)
Explore 8 Videos
- REDOX REACTIONSBook:AAKASH INSTITUTE ENGLISHChapter:REDOX REACTIONSExercise:Assignment (Section F) (Matrix-Match Type Questions)
Explore 4 Videos
- REDOX REACTIONSBook:AAKASH INSTITUTE ENGLISHChapter:REDOX REACTIONSExercise:Assignment (Section C) (Objective type Questions (More than one option is correct))
Explore 18 Videos
- PRINCIPLES OF QUALITATIVE ANALYSISBook:AAKASH INSTITUTE ENGLISHChapter:PRINCIPLES OF QUALITATIVE ANALYSISExercise:Assignment (SECTION H)
Explore 9 Videos
- SOLUTIONSBook:AAKASH INSTITUTE ENGLISHChapter:SOLUTIONSExercise:ASSIGMENT (SECTION-J) AAKASH CHALLENGERS QUESTIONS
Explore 10 Videos
Similar Questions
Redox is a reaction in which both oxidation and reduction will take place simultaneously . It is obvious that if one substance gives electron there must be another substance to accept these electrons . In some reactions, same substance is reduced as well as oxidised, these reactions are termed as disproportionation reactions.
For calculating equivalent mass in redox reaction change in oxidtaion number is realted to n-factor which is reciprocal of molar ratio.
Which of the following is an example of redox reaction ?
View SolutionRedox is a reaction in which both oxidation and reduction will take place simultaneously . It is obvious that if one substance gives electron there must be another substance to accept these electrons . In some reactions, same substance is reduced as well as oxidised, these reactions are termed as disproportionation reactions.
For calculating equivalent mass in redox reaction change in oxidtaion number is realted to n-factor which is reciprocal of molar ratio.
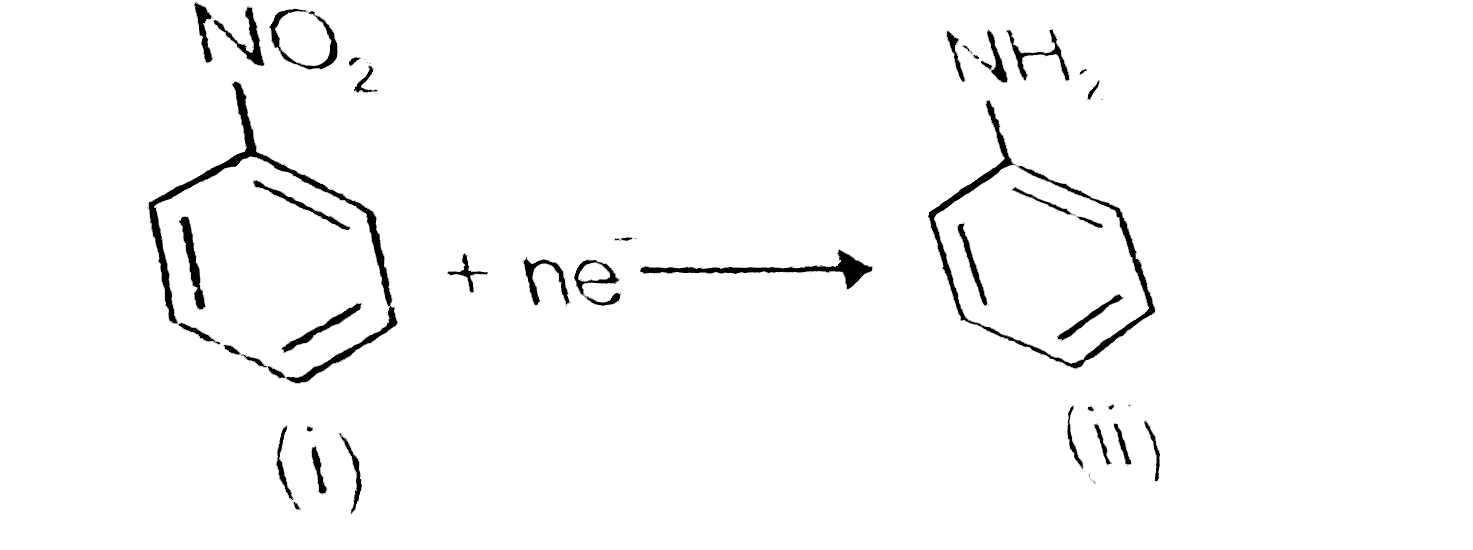
For converting one mole of nitrobenzene to aniline how many moles of electrons are transferred ?
View Solution
Knowledge Check
The reaction in which oxidation and reduction occurs simultaneously is called
AelectrolysisBredoxCoxidationDionisation
Redox is a reaction in which both oxidation and reduction will take place simultaneously . It is obvious that if one substance gives electron there must be another substance to accept these electrons . In some reactions, same substance is reduced as well as oxidised, these reactions are termed as disproportionation reactions.
For calculating equivalent mass in redox reaction change in oxidtaion number is realted to n-factor which is reciprocal of molar ratio.
This reaction is an example of
Br2+OH−→BrO−3+H2O+Br−
View SolutionRedox is a reaction in which both oxidation and reduction will take place simultaneously . It is obvious that if one substance gives electron there must be another substance to accept these electrons . In some reactions, same substance is reduced as well as oxidised, these reactions are termed as disproportionation reactions.
For calculating equivalent mass in redox reaction change in oxidtaion number is realted to n-factor which is reciprocal of molar ratio.
The equivalent weight of Cu2S in the following reaction is
Cu2S+O2→Cu+2+SO3
View SolutionRedox is a reaction in which both oxidation and reduction will take place simultaneously . It is obvious that if one substance gives electron there must be another substance to accept these electrons . In some reactions, same substance is reduced as well as oxidised, these reactions are termed as disproportionation reactions.
For calculating equivalent mass in redox reaction change in oxidtaion number is realted to n-factor which is reciprocal of molar ratio.
When P reacts with NaOH, the products are PH3 and NaH2PO2 which of the following statement is correct ?
View SolutionA _____ reaction is a reaction in which both oxidation and reduction take place simultaneously.
View SolutionIn which reaction, there is change in oxidation number of N atom?
View SolutionThe enthalpy of formation of hypothetical MgCl is −125kJmol−1 and for MgCl2 is −642kJmol−1. What is the enthalpy of the disproportionation of MgCl.
View SolutionWhich reaction does not represent auto redox or disproportionation ?
View Solution
