An ideal gas undergoes a process consisting of adiabatic and isothermal process only as shown in the figure. Then :
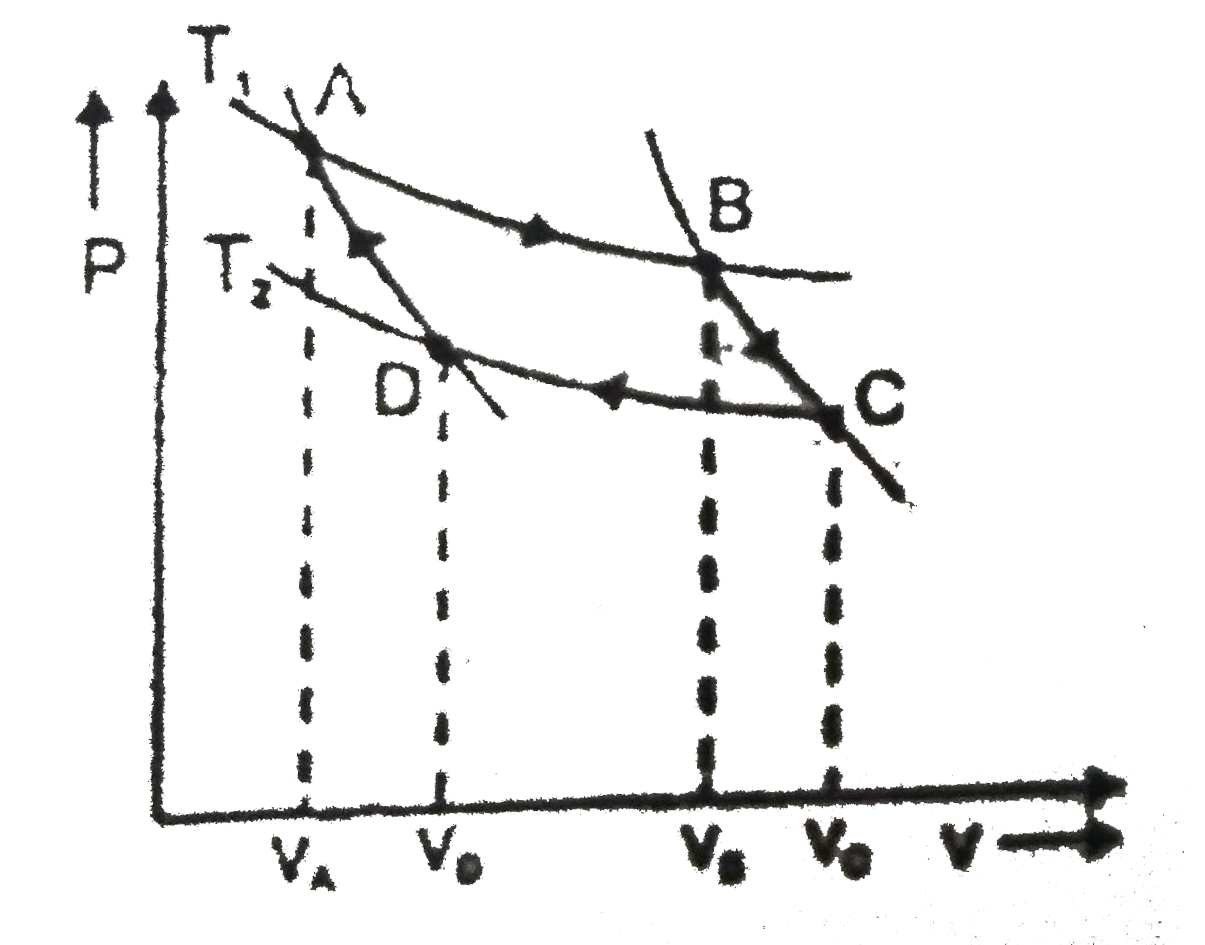
T1<T2
VAVC=VBVD
work done by the gas in process will be negative
efficiency =WAB+WBCQAB
The correct Answer is:B
PAVA=PBVB.....(1) (isothermal process)
PBVγB=PCVγC.......(2) (adiabatic process)
PCVC=PDVD......(3) (isothermal process)
PDVγD=PAVγA .......(4) (adiabatic process)
∴ multiplying all the above equations
PAPBPCVAVγBVCVγD=PAPBPCVγAVBVγCVD
Vγ−1BVγ−1D=Vγ−1AVγ−1C or VBVD=VAVC
टॉपर्स ने हल किए ये सवाल
समान प्रश्न
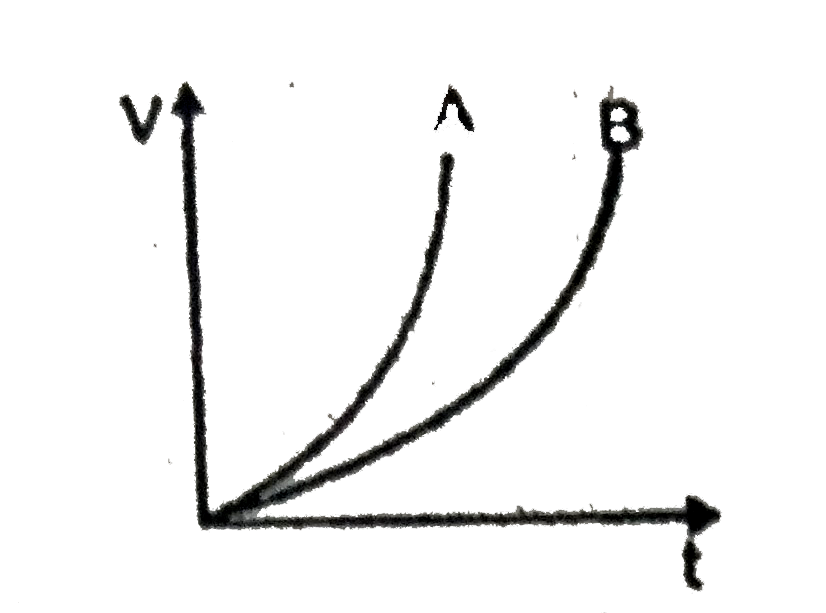
A velocity-time graph of two vehicals A & B starting from rest at the same time is given in the figure. The statements which is correct from graph.
View SolutionA particle performs SHM on x- axis with amplitude A and time period period T .The time taken by the particle to travel a distance A/5 string from rest is
View SolutionCan a process on an ideal gas be both adiabatic and isothermal. ?
View SolutionThe curves A and B in the figure shown P-V graphs for an isothermal and an adiabatic process for an idea gas. The isothermal process is represented by the curve A.
View SolutionDoes the internal energy of an ideal gas change in an isothermal process? In an adiabatic process?
View SolutionAn ideal gas undergoes a process consisting of adiabatic and isothermal process only as shown in the figure. Then :

View SolutionOne mole of an ideal gas is carried through a thermodynamics cycle as shown in the figure. The cycle consists of an isochoric, an isothermal and an adiabatic process. Find the adiabatic exponent of the gas.
View SolutionAn ideal gas system undergoes an isothermal process, then the work done during the process is
View SolutionAn ideal gas undergoes a cyclic process as shown in the given P-T diagram, where AC is adiabatic. The process is also represented by:
View SolutionAn ideal gas undergoes four processes: isochoric, isobaric, isothermal and adiabatic. In which process is (i) the change in internal energy zero?
View SolutionAn ideal gas undergoes four processes: isochoric, isobaric, isothermal and adiabatic. In which process is (ii) the work done zero?
View SolutionAn ideal gas undergoes four processes: isochoric, isobaric, isothermal and adiabatic. In which process is (iii) the heat exchange zero?
View SolutionAn ideal gas undergoes four different processes from the same initial state as shown in figure of P-V diagram. Four processes are adiabatic, isothermal, isobaric and isochoric. Out of 1,2,3 and 4 which one is adiabatic ?
View SolutionAn ideal gas system undergoes an isothermal process, then the work done during the process is
View SolutionCan a process on an ideal gas be both adiabatic and isothermal. ?
View Solution

