At what temperature will the molar kinetic energy of 0.3 mol of He be the same as that of 0.4 mol of argon at 400 K ?
533K
400K
346K
300K
The correct Answer is:1
[32nRT]He=32nRT
0.3T=0.4×400
T=533K
Topper's Solved these Questions
- GASEOUS STATEBOOK - RESONANCE ENGLISHCHAPTER - GASEOUS STATEEXERCISE - INORGANIC CHEMISTRY (COORDINATION COMPOUNDS)
36 Videos
- GASEOUS STATEBOOK - RESONANCE ENGLISHCHAPTER - GASEOUS STATEEXERCISE - ORGANIC CHEMISTRY(Hydrocarbon)
17 Videos
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS EXERCISE - Match the column
1 Video
- GENERAL ORGANIC CHEMISTRY IIBOOK - RESONANCE ENGLISHCHAPTER - GENERAL ORGANIC CHEMISTRY IIEXERCISE - Part-III: Section-5: Matching List Type
1 Video
Similar Questions
There are 6.02×1022 molecules each of N2,O2 and H2 which are mixed together at 760mm and 273K. The mass of the mixture in grams is :
View SolutionA certain quantity of a gas occupied 100ml when collected over water at 15∘C and 750mm pressure . It occupies 91.9ml in dry state at NTP. Find the V.P. of water at 15∘C
View SolutionCuSO4 reacts with NH3 to give blue complex of
View SolutionMatch List I with List II and select the correct answer using the codes given below the lists
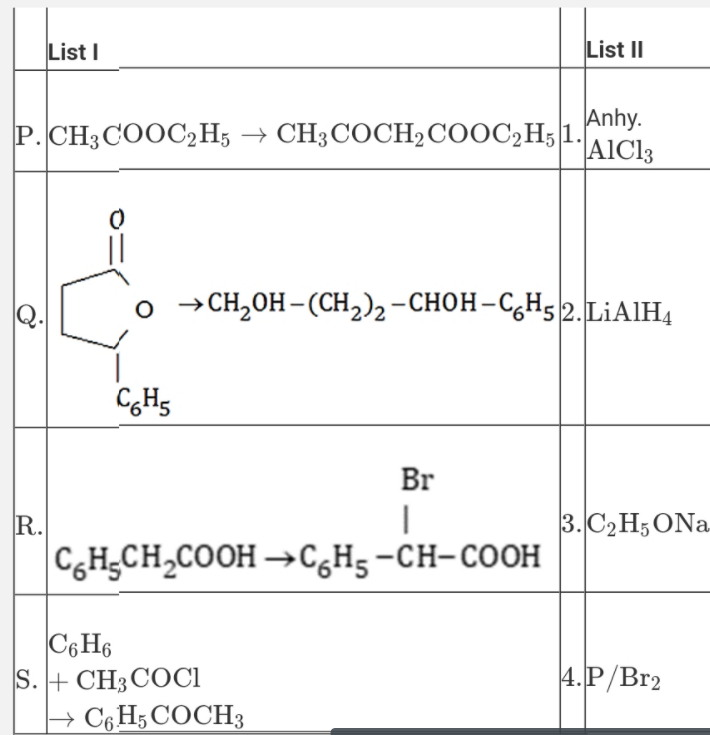
View SolutionAt what temperature will the molar kinetic energy of 0.3 mol of 'He' be the same as that of 0.4 mol of argon at 400 K ?
View SolutionAt what temperature will the total KE of 0.3mol of He be the same as the total KE of 0.40mol of Ar at 400K
View SolutionAt what temperature will the total KE of 0.3 mol of He be the same as the total KE of 0.40 mol of Ar at 400K ?
View SolutionAt what temperature will the total KE of 0.3 mo of He be the same as the total KE of 0.40 mol of Ar at 400K?
View SolutionWhat is the temperaure at which the kinetic energy of 0.3 mole of helium is equal tp the kinetic energy of 0.4 mole of argon at 400K
View SolutionAt what temperature will the total kinetic energy of 0.30 mol of helium be same as the total kinetic energy of 0.40 mol of argon at 500 K.
View SolutionWhat is the activation energy (KJ/mol) for a reaction if its rate constant doubles when the temperature is raised from 300 K to 400 K ? (R=8.314 J mol−1K−1)
View SolutionIf the kinetic energy in j, of CH4 (molar mass =16gmol−1) at T (K) is X, the kinetic energy in j , of O2 (molar mass = 32 g mol−1) at the same temperature is
View SolutionWhat is the temperature at which the kinetic energy of 0.3 moles of helium is equal to the kinetic energy of 0.4 moles of argon at 400 K. ?
View SolutionAt what temperature will the total kinetic energy of 0.30 mol of helium be same as the total kinetic energy of 0.40 mol of argon at 500 K.
View SolutionIf the kinetic energy in j, of CH4 (molar mass =16gmol−1) at T (K) is X, the kinetic energy in j , of O2 (molar mass = 32 g mol−1) at the same temperature is
View SolutionAt what temperature will the molar kinetic energy of 0.3 mol of 'He' be the same as that of 0.4 mol of argon at 400 K ?
View Solution

