There are 6.02×1022 molecules each of N2,O2 and H2 which are mixed together at 760mm and 273K. The mass of the mixture in grams is :
6.2
4.12
3.09
7
The correct Answer is:1
To solve the problem step by step, we will calculate the mass of the mixture of gases (N₂, O₂, and H₂) given the number of molecules of each gas, the pressure, and the temperature.
Step 1: Determine the number of moles of each gas
We are given that there are 6.02×1022 molecules of each gas. We know that Avogadro's number is approximately 6.023×1023 molecules per mole.
To find the number of moles (n) of each gas, we use the formula:
n=Number of moleculesAvogadro's number
Substituting the values:
n=6.02×10226.023×1023≈0.1 moles
Step 2: Calculate the molar mass of each gas
Next, we need to find the molar mass of each gas:
- Molar mass of N2 (Nitrogen) = 28 g/mol
- Molar mass of O2 (Oxygen) = 32 g/mol
- Molar mass of H2 (Hydrogen) = 2 g/mol
Step 3: Calculate the mass of each gas
Now, we can calculate the mass of each gas using the formula:
Mass=Number of moles×Molar mass
For each gas:
1. Mass of N2:
MassN2=0.1 moles×28 g/mol=2.8 g
2. Mass of O2:
MassO2=0.1 moles×32 g/mol=3.2 g
3. Mass of H2:
MassH2=0.1 moles×2 g/mol=0.2 g
Step 4: Calculate the total mass of the mixture
Now, we can find the total mass of the mixture by adding the masses of all three gases:
Total mass=MassN2+MassO2+MassH2
Total mass=2.8 g+3.2 g+0.2 g=6.2 g
Final Answer
The mass of the mixture in grams is 6.2 g.
---
We are given that there are 6.02×1022 molecules of each gas. We know that Avogadro's number is approximately 6.023×1023 molecules per mole.
Next, we need to find the molar mass of each gas:
- Molar mass of O2 (Oxygen) = 32 g/mol
- Molar mass of H2 (Hydrogen) = 2 g/mol
Now, we can calculate the mass of each gas using the formula:
MassN2=0.1 moles×28 g/mol=2.8 g
MassO2=0.1 moles×32 g/mol=3.2 g
MassH2=0.1 moles×2 g/mol=0.2 g
Now, we can find the total mass of the mixture by adding the masses of all three gases:
Total mass=2.8 g+3.2 g+0.2 g=6.2 g
The mass of the mixture in grams is 6.2 g.
টপার এই প্রশ্নগুলো সমাধান করেছে
- GASEOUS STATEBook:RESONANCE ENGLISHChapter:GASEOUS STATEExercise:ORGANIC CHEMISTRY(Hydrocarbon)
Explore 17 Videos
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS Book:RESONANCE ENGLISHChapter:D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS Exercise:Match the column
Explore 1 Video
- GENERAL ORGANIC CHEMISTRY IIBook:RESONANCE ENGLISHChapter:GENERAL ORGANIC CHEMISTRY IIExercise:Part-III: Section-5: Matching List Type
Explore 1 Video
সম্পর্কিত প্রশ্ন
A certain quantity of a gas occupied 100ml when collected over water at 15∘C and 750mm pressure . It occupies 91.9ml in dry state at NTP. Find the V.P. of water at 15∘C
View SolutionCuSO4 reacts with NH3 to give blue complex of
View SolutionMatch List I with List II and select the correct answer using the codes given below the lists
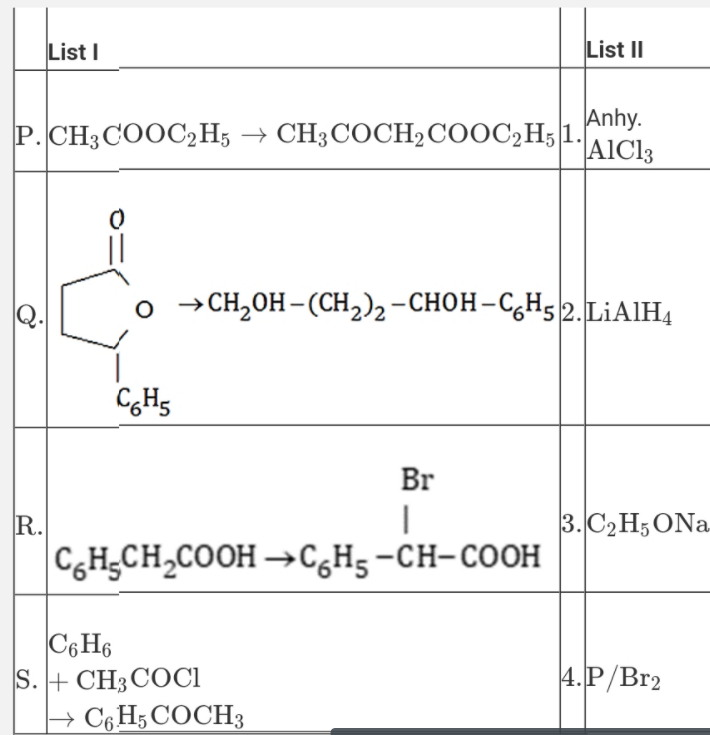
View SolutionA gaseous mixture contains oxygen and nitrogen in the ratio of 1:8 by mass. The ratio of their respective number of molecules (N_(O_(2)):N_(H_(2))) is
View SolutionThere are 6.02 xx 10^(22) molecules each of N_(2),O_(2) and H_(2) which are mixed together at 760 mm and 273 K . The mass of the mixture in grams is :
View SolutionVolume of N_(2) at 1 atm, 273 K required to form a monolayer on the surface of iron catalyst is 8.15ml//gm of the adsorbent. What will be the surface area of the adsorbent per gram if each nitrogen molecule occupies 16 xx 10^(-22)m^(2) ? [Take : N_(A)=6xx10^(23) ]
View Solutionगैसों का एक मिश्रण, जिसमे 65%N2,15%O2 तथा 20%CO2 है, का दाब 760 मिमी Hg है। प्रत्येक गैस का आंशिक दाब ज्ञात करो।
View Solutionऑक्सीजन के 16 ग्राम तथा हाइड्रोजन के 3 ग्राम को मिलाया गया और 760 मिमी दाब तथा 273 पर एक बर्तन में रखा गया। मिश्रण के द्वारा घेरा गया कुल आयतन..... लीटर होगा।
View Solutionकिसी गैसीय मिश्रण के 2 लीटर में 4.0 ग्राम H2 तथा 5.6 ग्राम N2 हैं। इनके सक्रिय द्रव्यमानों कि गड़ना कीजिये।
View SolutionWhat is the mass in grams of one molecule of caffeine (C_(8)H_(10)N_(4)O_(2)) ?
View Solutionनिम्नलिखित का द्रव्यमान ग्राम में ज्ञात कीजिए -
12 मोल N2(ii) 3ग्राम अणु O2 |
View Solutionनिम्नलिखित का ग्राम में द्रव्यमान ज्ञात करो -
(a) 2 ग्राम-अणु O2 (b) 2 ग्राम-अणु N2 |
View Solutionनिम्नलिखित में मोलों की गणना करो ।
(i) 3.01×1023CO2 अणु , (ii) 6.02×1021H2O अणु
View SolutionThe number of gram molecules of oxygen in 6.02×1024 water molecules is ________ .
View SolutionThe number of gram molecules of oxygen in 6.02 xx 10^24 CO molecules is :
View SolutionThe average speed of \(H_{2}\),\(N_{2}\) and \(O_{2}\) gas molecules is in the order \(H_{2} > N_{2} > O_{2}\) \(O_{2} > N_{2} > H_{2}\) \(H_{2} > O_{2} > N_{2}\) \(N_{2} > O_{2} > H_{2}\)
View Solution
