
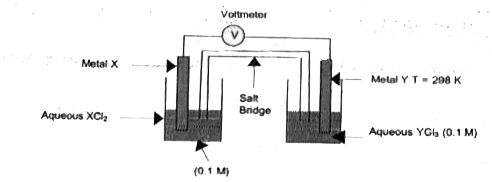
The following diagram shows the setup of an electrochemical cell in which the respective half cells contain aqueous 1.0 M solutions of the salts XCl2 and YCl3. Given that
3X(s)+2Y3+(aq)→3X2+(aq)+2Y(s)EcellL<0 Which of the following statements is correct?
The electrode made from metal X has positive
Electrode Y is te anode
The flow of electrons is from Y to X
lons move randomly in salt bridge here.
The correct Answer is:D
Look carefully at the setup. The connecting wire is broken & circuit is incomplete, no reaction occurs.
Similar Questions
The following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl2andYCl3. Given that
3X(s)+2Y3+(aq)→3X2(aq)+2Y(s)εcell>0
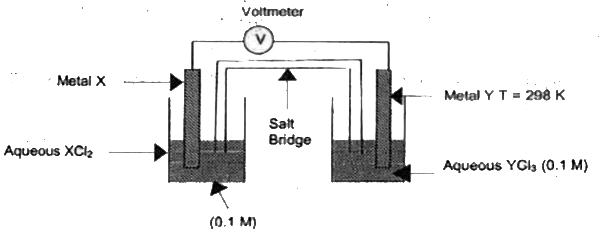
Which of the following statements is correct?
I. The electrode made from metal X has positive polarity
II. Electrode Y Is the anode
II. Tho flow of electrons is from Y to X
IV. The reaction at electrode X is an oxidation
V. The salt bridge would most likely contain silver nitrate
View SolutionThe following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl2andYCl3. Given that
3X(s)+2Y3+(aq)→3X2(aq)+2Y(s)εcell>0
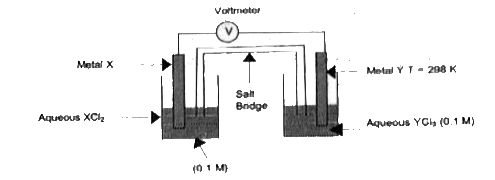
if salt-bridge is removed:
View SolutionThe following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl2andYCl3. Given that
3X(s)+2Y3+(aq)→3X2(aq)+2Y(s)εcell>0
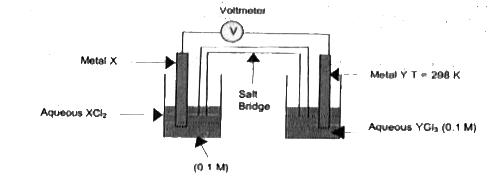
Cell reaction is non spontaneous:
View SolutionThe following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl2andYCl3. Given that
3X(s)+2Y3+(aq)→3X2(aq)+2Y(s)εcell>0
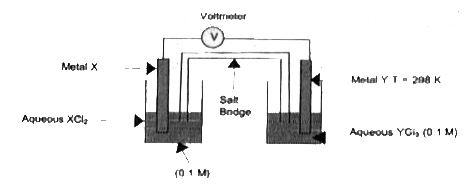
Ecell of the above designed cell is if standard emf of cell is -0.20 V.
View SolutionThe following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl2andYCl3. Given that
3X(s)+2Y3+(aq)→3X2+(aq)+2Y(s)εcell>0
E∘X/X2+=0.20v,E∘Y/Y3+=0.40V the E∘cell of the above designed cell is
View SolutionFor the cell (at 298K)
Ag(s)|AgCl(s)|Cl−(aq)∣|AgNO3(aq)|Ag(s)
Which of the following is correct?
View SolutionWhich one of the following condition will increase the voltage of the cell represented by the equation?
Cu(s)+2Ag+(aq) (Cu2+(aq)+2Aq(s)
View SolutionThe equillibrium constant for thefollowing general reaction is 1030. Calculate E∘ for the cell at 298 K.
2X2(s)+3Y2+(aq)→2X3+2(aq)+3Y(s)
View SolutionE.M.F. of Ni(s)∣∣Ni2+(aq)∣∣∣∣Cu2+(aq)∣∣Cu(s) cell can be increased by
View SolutionWrite the cell reaction for each of the following cells.
Ni(s)∣∣Ni2+(aq)∣∣∣∣Cu2+(aq)∣∣Cu(s).
View Solution
