The following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl2andYCl3. Given that
3X(s)+2Y3+(aq)→3X2(aq)+2Y(s)εcell>0
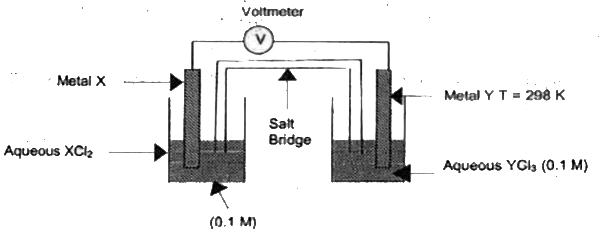
Which of the following statements is correct?
I. The electrode made from metal X has positive polarity
II. Electrode Y Is the anode
II. Tho flow of electrons is from Y to X
IV. The reaction at electrode X is an oxidation
V. The salt bridge would most likely contain silver nitrate
only I
only I & IV
only V
all except V
The correct Answer is:B
Step by step video solution for The following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl_(2) and YCl_(3). Given that 3X(s)+2Y^(3+) (aq) to 3X^(2)(aq)+2Y(s) epsi_("cell") gt 0 Which of the following statements is correct? I. The electrode made from metal X has positive polarity II. Electrode Y Is the anode II. Tho flow of electrons is from Y to X IV. The reaction at electrode X is an oxidation V. The salt bridge would most likely contain silver nitrate by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
The following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl2andYCl3. Given that
3X(s)+2Y3+(aq)→3X2(aq)+2Y(s)εcell>0
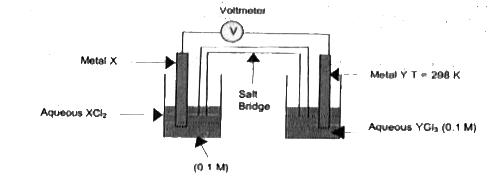
Cell reaction is spontaneous:
View SolutionWhich of the following reactions occurs at anode in the electrochemical cell reaction ? Zn(s)+Cu2+(aq)→Zn2+(aq)+Cu(s)
View Solutiona) For the electrochemical cell represented as: Cu(s)∣∣Cu2+(aq)∣∣∣∣Ag+(aq)∣∣Ag(s), write the half cell reaction that occurs at (i) anode (ii) cathode
View SolutionA cell utilises the following reaction: 2CO^(3+) (aq)+Zn(s) to 2CO^(2+)(aq)+Zn^(2+) (aq) What is the effect on cell emf of each of the following changes? The size or area of Zn (s) electrode is doubled
View SolutionA cell utilises the following reaction: 2CO^(3+) (aq)+Zn(s) to 2CO^(2+)(aq)+Zn^(2+) (aq) What is the effect on cell emf of each of the following changes? The size or area of Zn (s) electrode is doubled
View SolutionA cell is represented in the following way. Cu(s)|Cu^(2+)(aq)||Ag^+(aq)|Ag(s) Which electrode acts as anode?
View SolutionThe following diagram shows the setup of an electrochemical cell in which the respective half cells contain aqueous 1.0 M solutions of the salts XCl_(2) and YCl_(3) . Given that 3X(s)+2Y^(3+)(aq)to3X^(2+)(aq)+2Y(s)E_(cell)Llt0 Which of the following statements is correct?
View SolutionCalculate the standard cell potentials of the cells in the following reactions: (i) 2Cr(s)+3Cd^(2+)(aq)to2Cr^(3+)(aq)+3Cd (ii) Fe^(2+)(Aq)+Ag^(+)(aq)toFe^(3+)(Aq)+Ag(s)
View Solution
