The maximum oxidation state shown by V(Z=23),Cr(Z=24),Co(Z=27),Sc(Z=21), are respectively
3
4
10
11
The correct Answer is:2
C+211H22O11→CO2
⇒pH of Acid=pOH of base =14−pH of base ⇒pOH cf base =14−4=10
Topper's Solved these Questions
- D & F BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:D & F BLOCK ELEMENTSExercise:INORGANIC CHEMISTRY(d & f- Block Elments)
Explore 40 Videos
- D & F BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:D & F BLOCK ELEMENTSExercise:ORGANIC CHEMISTRY(Aldehydes , Ketones, Carboxylic acid)
Explore 15 Videos
- COORDINATION COMPOUNDSBook:RESONANCE ENGLISHChapter:COORDINATION COMPOUNDSExercise:Additional Problem for Self Practice (APSP) Part-IV Practice Test -2 (Section-5) (Matching Lift type )
Explore 1 Video
- D BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:D BLOCK ELEMENTSExercise:EXERCISE-3 PART-III
Explore 37 Videos
Similar Questions
The number of photons of light of ¯v=3.5×106m−1 necessary to provide 2 J of energy are:
View SolutionThe number of photons of light of ¯v=3.5×106m−1 necessary to provide 2 J of energy are:
View SolutionColumn-I and Column-II contains four entries each. Entries of Column-I are to be matched with some entries of Column-II One or more than one entries of Column-I may have the matching with the same entries of Column-II.
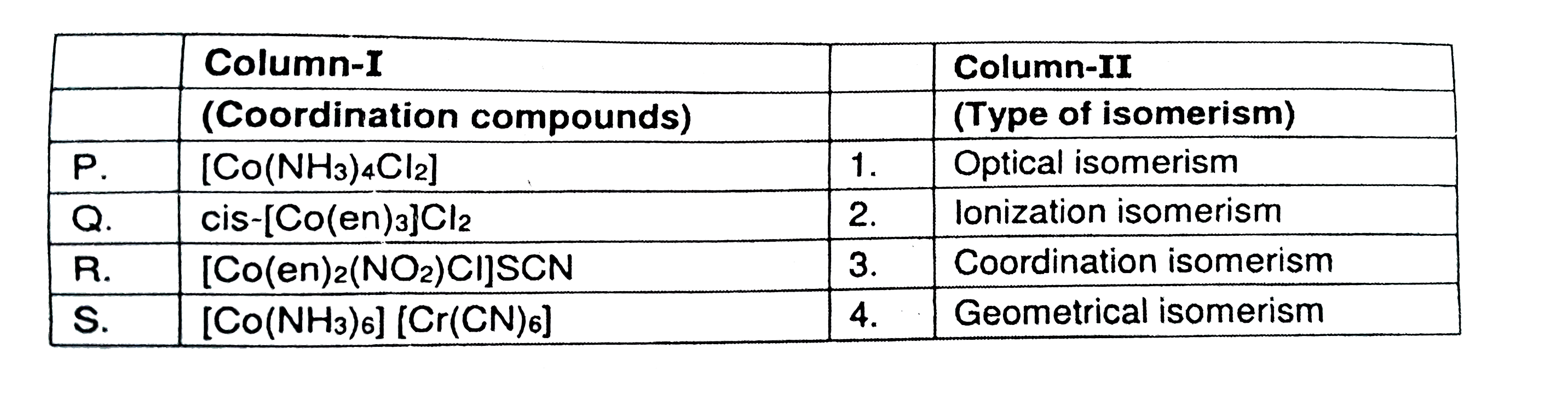
View SolutionOf the ions Ag+,Co2+ and Ti4+ ,which ones will be coloured in aqueous solutions. (Atomic numbers:)
Ag = 47 , Co = 27, Ti = 22)
(b) If each one of the above ionic species is in turn placed in magnetic field, how will it respond and why?
View SolutionMagnetic moment of V(Z = 23),Cr(Z = 24),and Mn(Z= 25) are x,y,z repectively hence
View SolutionThe maximum oxidation state shown by V(Z=23),Cr(Z=24),Co(Z=27),Sc(Z=21), are respectively
View SolutionAmong V(Z=23),Cr(Z=24),Mn(Z=25) which will have highest magnetic moment.
View SolutionMagnetic moments of V(Z = 23), Cr(Z = 24), Mn (Z = 25) are x,y,z. HencE:
View SolutionOut of the following transition elements, the maximum number of oxidation states are shown by
(a) Sc(Z=21)
(b) Cr (Z=24)
(c) Mn (Z=25)
(d) Fe (Z=26)
View SolutionSc (Z=21) is a transition element but Zinc (Z=30) is not because
View SolutionSc (Z=21) is a transition elements but Zn(Z=30) is not because….
View SolutionOut of the following transition elements, the maximum number of oxidation states are shown by
(a) Sc(Z=21)
(b) Cr (Z=24)
(c) Mn (Z=25)
(d) Fe (Z=26)
View SolutionAmong V(Z=23) , Cr(Z=24) , Mn(Z=25) which will have the highest magnetic moment?
View SolutionWhich of the following is paramagnetic ?
Sc3+(Z=21),Cu+(Z=29)
View SolutionMagnetic moments of V(Z=23)Cr(Z=24) Mn(Z=25) are x,y,z hence
View SolutionMagnetic moment of V(Z=23),Cr(Z=24),andMn(Z=25)arex,y,zrepectively hence
View Solution
