Yellow colour of chromates changes to orange on acidification due to formation of:
10−pH+10−pOH=10−14
pHα1[H+]
Kw∝T
dissociation constant of water K=1.8×10−16
The correct Answer is:1
(1) 10−pH×10−pOH=10−Kw
(2) as [H+] increases, pH decreases,
(3) since ionization of water is endothermic in nature hence increase in temperature increases Kw.
(4) Ka of H2O=[H+][OH−][H2O]=10−1455.55=1.8×10−16
10−pH×10−pOH=10−Kw
Topper's Solved these Questions
- D & F BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:D & F BLOCK ELEMENTSExercise:INORGANIC CHEMISTRY(d & f- Block Elments)
Explore 40 Videos
- D & F BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:D & F BLOCK ELEMENTSExercise:ORGANIC CHEMISTRY(Aldehydes , Ketones, Carboxylic acid)
Explore 15 Videos
- COORDINATION COMPOUNDSBook:RESONANCE ENGLISHChapter:COORDINATION COMPOUNDSExercise:Additional Problem for Self Practice (APSP) Part-IV Practice Test -2 (Section-5) (Matching Lift type )
Explore 1 Video
- D BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:D BLOCK ELEMENTSExercise:EXERCISE-3 PART-III
Explore 37 Videos
Similar Questions
The number of photons of light of ¯v=3.5×106m−1 necessary to provide 2 J of energy are:
View SolutionThe number of photons of light of ¯v=3.5×106m−1 necessary to provide 2 J of energy are:
View SolutionColumn-I and Column-II contains four entries each. Entries of Column-I are to be matched with some entries of Column-II One or more than one entries of Column-I may have the matching with the same entries of Column-II.
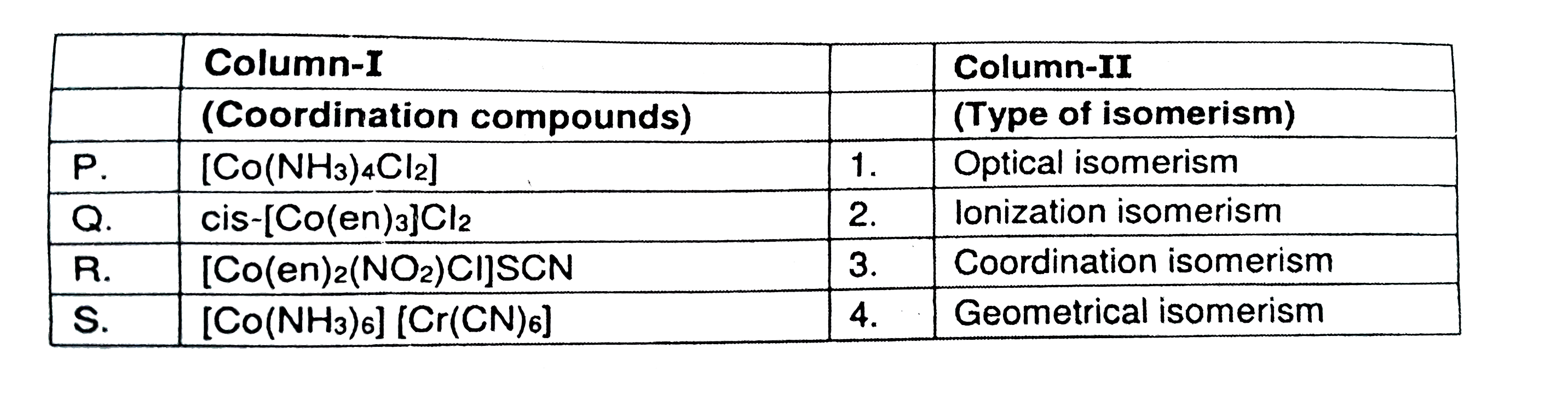
View SolutionOf the ions Ag+,Co2+ and Ti4+ ,which ones will be coloured in aqueous solutions. (Atomic numbers:)
Ag = 47 , Co = 27, Ti = 22)
(b) If each one of the above ionic species is in turn placed in magnetic field, how will it respond and why?
View SolutionThe orange colour of dichromate solution changes of blue on heating with alkalies due to the formation of chromate ions.
View SolutionAssertion: If a strong acid is added to a solution of potassium chromate it changes its colour from yellow to orange. Reason: The colour change is due to the oxidation of potassium chromate.
View SolutionThe yellow colour of chromates changes to orange on acidification due to the formation of
View SolutionThe yellow colour of chromates changes to orange on acidification due to formation of:
View SolutionThe yellow colour solution of Na2CrO4 changes to orange red on passing CO2 gas due to the formation of :-
View SolutionStatement-1. To a solution of potassium chromate, if a strong acid is added, it changes its colour from yellow to orange. Statement-2. The colour change is due to the change in the oxidation state of potassium chromate.
View SolutionThe yellow colour of chromates changes to orange on acidification due to the formation of
View SolutionStatement 1 : To a solution of potassium chromate if a strong acid is added it changes its colour from yellow to orange. Statement 2 : The colour change is due to the oxidation of potassium chromate.
View SolutionThe yellow coloured solution of chromate salt changes to orange colour on acidification due to the formation of :
View SolutionThe orange colour of dichromate solution changes of blue on heating with alkalies due to the formation of chromate ions.
View SolutionThe yellow colour of chromates changes to orange on acidification due to formation of:
View SolutionYellow colour of chromates changes to orange on acidification due to formation of:
View Solution
