(a) Transition metals can act as catalysts because these can change their oxidation state. How does Fe (III) catalyse the reaction between iodide and persulphate ions?
(b) Mention any three processes where transition metals act as catalysts.
Acid
Basic
Neutral
None of these
The correct Answer is:2
Step by step video solution for (a) Transition metals can act as catalysts because these can change their oxidation state. How does Fe (III) catalyse the reaction between iodide and persulphate ions? (b) Mention any three processes where transition metals act as catalysts. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- D & F BLOCK ELEMENTSBOOK - RESONANCE ENGLISHCHAPTER - D & F BLOCK ELEMENTSEXERCISE - INORGANIC CHEMISTRY(d & f- Block Elments)
40 Videos
- D & F BLOCK ELEMENTSBOOK - RESONANCE ENGLISHCHAPTER - D & F BLOCK ELEMENTSEXERCISE - ORGANIC CHEMISTRY(Aldehydes , Ketones, Carboxylic acid)
15 Videos
- COORDINATION COMPOUNDSBOOK - RESONANCE ENGLISHCHAPTER - COORDINATION COMPOUNDSEXERCISE - Additional Problem for Self Practice (APSP) Part-IV Practice Test -2 (Section-5) (Matching Lift type )
1 Video
- D BLOCK ELEMENTSBOOK - RESONANCE ENGLISHCHAPTER - D BLOCK ELEMENTSEXERCISE - EXERCISE-3 PART-III
37 Videos
Similar Questions
The number of photons of light of ¯v=3.5×106m−1 necessary to provide 2 J of energy are:
View SolutionThe number of photons of light of ¯v=3.5×106m−1 necessary to provide 2 J of energy are:
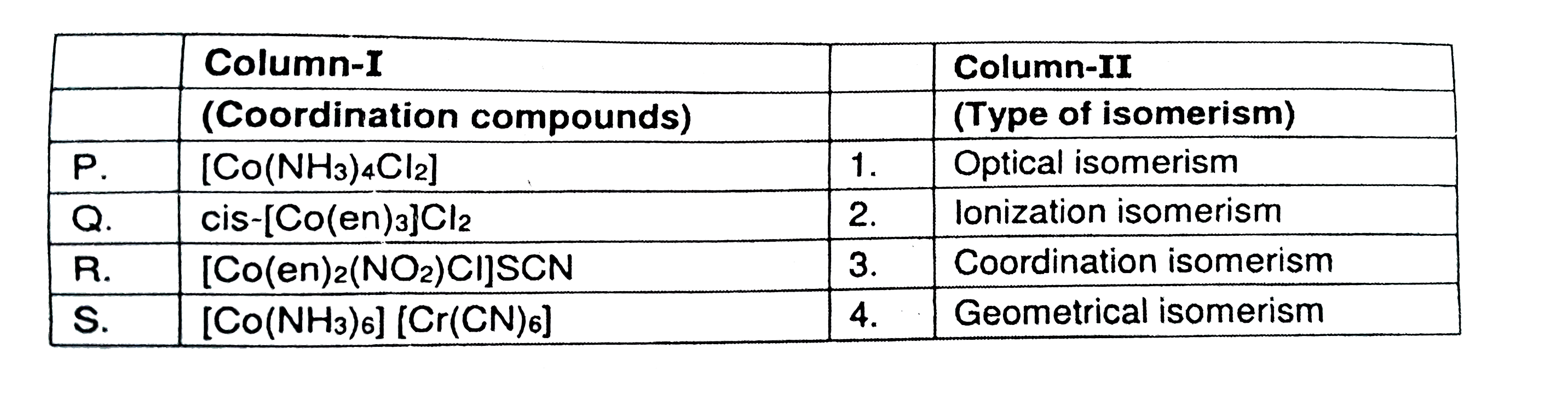
View SolutionColumn-I and Column-II contains four entries each. Entries of Column-I are to be matched with some entries of Column-II One or more than one entries of Column-I may have the matching with the same entries of Column-II.
View SolutionOf the ions Ag+,Co2+ and Ti4+ ,which ones will be coloured in aqueous solutions. (Atomic numbers:)
Ag = 47 , Co = 27, Ti = 22)
(b) If each one of the above ionic species is in turn placed in magnetic field, how will it respond and why?
View SolutionGenerally transition metals act as catalyst because of
View Solution(a) Transition metals can act as catalysts because these can change their oxidation state. How does Fe (III) catalyse the reaction between iodide and persulphate ions? (b) Mention any three processes where transition metals act as catalysts.
View SolutionAssertion : Iron(III) catalyses the reaction between iodide and persulphate ions. Reason : Transition metals act as catalysts.
View SolutionWhich of the following transition metal ion catalyses the reaction between iodide and persulphate ions?
View SolutionWrite down the name and formula of an oxide of a transition metal which acts as a catalyst.
View SolutionTransition metals can act as catalysts because these can change their oxidation states. How does Fe(III) catalyse the reaction between iodide and persulphate ions ?
View SolutionMention any three processes where transition metals acts as catalysts.
View Solution(a) Transition metals can act as catalysts because these can change their oxidation state. How does Fe(III) catalyse the reaction between iodide and persulphate ions ? (b) Mention any three processes where transition metals act as catalysts.
View Solutionअभिकथनः आयरन (III), आयोडाइड एवं परसल्फेट आयनों के मध्यम अभिक्रिया को उत्प्रेरित करता हैं।
तर्कः संक्रमण धातुएँ उत्प्रेरक की भाँति कार्य करती है।
View SolutionWhy transition metals act as good catalysts ?
View Solution(a) Transition metals can act as catalysts because these can change their oxidation state. How does Fe(III) catalyse the reaction between iodide and persulphate ions?
(b) Mention any three processes where transition metals act as catalysts.
View Solution(a) Transition metals can act as catalysts because these can change their oxidation state. How does Fe (III) catalyse the reaction between iodide and persulphate ions?
(b) Mention any three processes where transition metals act as catalysts.
View Solution

