Four successive members of the first row transition elements are listed below with their atomic numbers. Which one of them is expected to have the highest third ionisation enthalpy ?
Cr(Z=24)
Mn(Z=25)
Fe(Z=26)
Co(Z=27)
The correct Answer is:D
Step by step video solution for Four successive members of the first row transition elements are listed below with their atomic numbers. Which one of them is expected to have the highest third ionisation enthalpy ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS EXERCISE - Additional Problems for Self practice (APSP)
30 Videos
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS EXERCISE - National Standard Examination In chemistry
35 Videos
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS EXERCISE - Exercise 2
40 Videos
- CHEMICAL EQUILIBRIUMBOOK - RESONANCE ENGLISHCHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - Advanced Level Problems (Part-3)(Stage-5)
2 Videos
- GASEOUS STATEBOOK - RESONANCE ENGLISHCHAPTER - GASEOUS STATEEXERCISE - ORGANIC CHEMISTRY(Hydrocarbon)
17 Videos
Similar Questions
The maximum oxidation state shown by V(Z=23),Cr(Z=24),Co(Z=27),Sc(Z=21), are respectively
View SolutionThe aqueous solution of transition metal salt changes colour from pink to blue, when concentrated hydrochloric acid is added to it. The changes in colour is due to:
View SolutionMatch the compounds of column X with oxidation state of central atom in column Y.
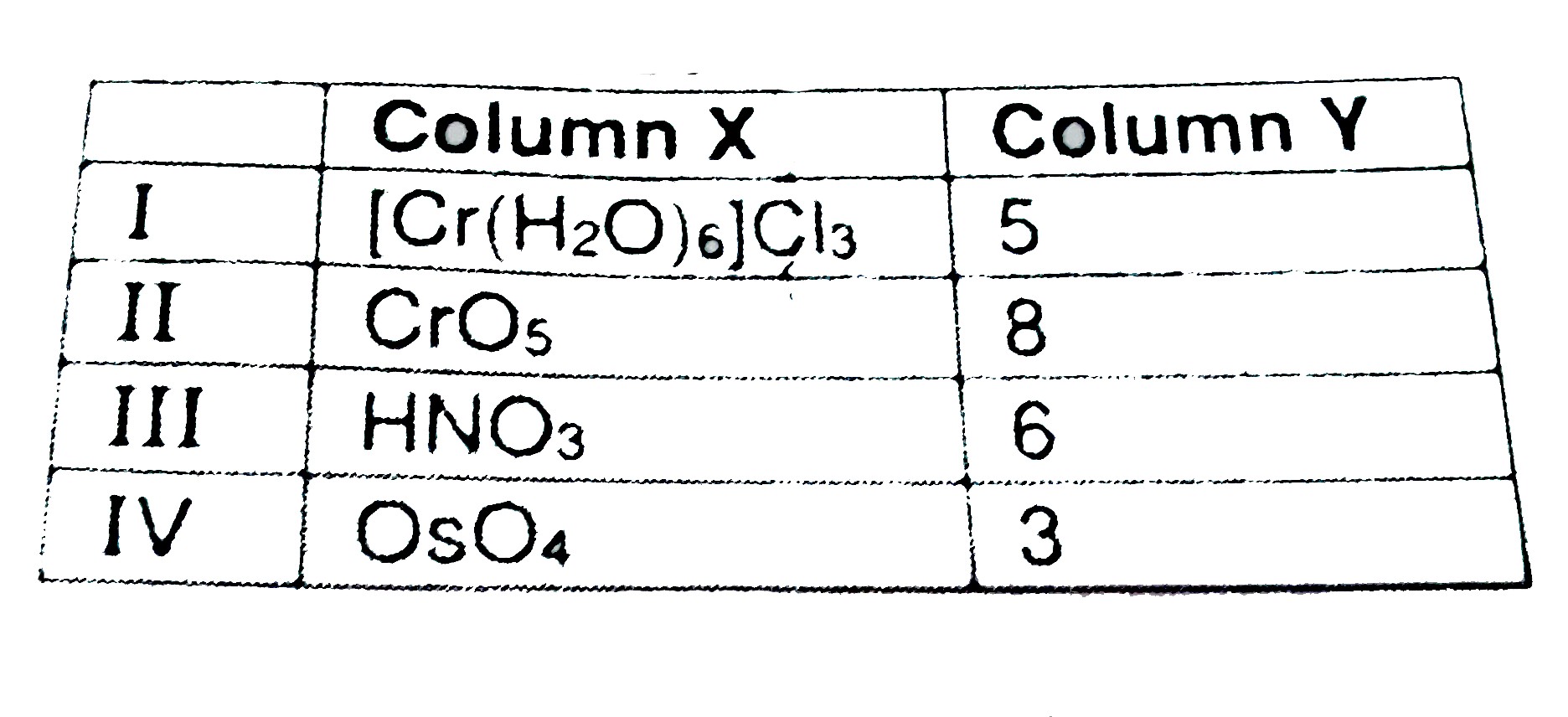 .
.
View SolutionAn alloy consists of rubidium and one of the other alkali metals. A sample of 4.6g of the alloy when allowed to react with water, liberates 2.241dm3 of hydrogen at STP.
Relative atomic masses: AT(Li)=7,AT(Na)=23,AT(K)=39,AT(Rb)=85.5,AT(Cs)=1.33
Which alkali metal is the component of the alloy?
View SolutionA certain quantity of a gas occupied 100ml when collected over water at 15∘C and 750mm pressure . It occupies 91.9ml in dry state at NTP. Find the V.P. of water at 15∘C
View SolutionFour successive members of the first row transition elements are listed below with their atomic number. Which one of them is expected to have the highest third ionisation enthalpy ?
View SolutionFour successive members of the first row transition elements are listed below with atomic numbers. Which one of them is expected to have the highest E0M3+/M2+ value
View SolutionFour successive members of the first row transition elements are listed below with their atomic number. Which one of them is expected to have the highest third ionisation enthalpy ?
View SolutionFour successive members of the first row transition elements are listed below with atomic numbers. Which one of them is expected to have the highest E0M3+/M2+ value
View SolutionFour successive members of the first row transition elements are listed below with atomic numbers. Which one of them is expected to have the highest E0M3+∣M2+ value -
View SolutionFour sucessive members of the first two transition elements are listed below with their atomic numbers. Which one of them is expected to have highest third ionisation enthalpy ?
View Solutionप्रथम पंक्ति के संक्रमण तत्वों के चार क्रमिक सदस्यों को उनके परमाणु क्रमांक के साथ नीचे सूचीबद्ध किया गया है। उनमें से किसमें अधिकतम तृतीय आयनन एन्थैल्पी अपेक्षित की जाती है
View SolutionFour successive members of the first row transition elements are listed ahead with atomic numbers. Which one of them is expected to have the highest E∘(M3+∣M2+) value?
View Solutionप्रथम श्रेणी के संक्रमण तत्वों के चार सदस्यों को क्रम से उनकी परमाणु संख्याओं के साथ नीचे सूचीबद्ध किया जाता है। इनमें से किसकी तृतीय आयनन एन्थैल्पी सर्वाधिक अपेक्षित है।
View SolutionFour successive members of the first row transition elements are listed below with their atomic number. Which one of them is expected to have the highest third ionisation enthalpy ?
View SolutionFour successive member of the first row tran sition elements are listed below with their atomic numbers. Which one of them is expected to have the highest third ionisation enthalpy?
View Solution

