Which of the following statements is false?
Na2Cr2O7 is less soluble tha K2Cr2O7
Na2Cr2O7 is primary standard in volumentry
CrO2−4 is tetrahedral in shape
Cr2O27 has a Cr-O-Cr bond
The correct Answer is:A
To determine which of the statements is false regarding the compounds Na2Cr2O7 and K2Cr2O7, we can analyze each statement step by step.
1. Analyze Statement A: Na2Cr2O7 is less soluble than K2Cr2O7.
- Sodium (Na) and potassium (K) are both alkali metals.
- As we move down the group in the periodic table, the size of the cations increases.
- Na+ is smaller than K+, which means Na+ can be more effectively surrounded by water molecules (hydration).
- Therefore, Na2Cr2O7 is actually more soluble than K2Cr2O7, making Statement A false.
2. Analyze Statement B: Na2Cr2O7 is a primary standard in volumetry.
- A primary standard must have a stable composition and not absorb moisture from the air.
- Na2Cr2O7 is hygroscopic, meaning it absorbs moisture, which affects its weight and concentration.
- Thus, Na2Cr2O7 cannot be considered a primary standard, making Statement B false.
3. Analyze Statement C: CrO4^2- is tetrahedral in shape.
- The chromate ion (CrO4^2-) has a central chromium atom surrounded by four oxygen atoms.
- The hybridization of Cr in CrO4^2- is sp3, leading to a tetrahedral geometry.
- Therefore, Statement C is true.
4. Analyze Statement D: Cr2O7^2- has a Cr-O-Cr bond.
- The dichromate ion (Cr2O7^2-) consists of two chromium atoms connected by oxygen atoms.
- The structure indeed includes Cr-O-Cr bonds.
- Thus, Statement D is true.
Conclusion:
- The false statements are A and B.
Final Answer:
The false statements are:
- A: Na2Cr2O7 is less soluble than K2Cr2O7.
- B: Na2Cr2O7 is a primary standard in volumetry.
---
- Sodium (Na) and potassium (K) are both alkali metals.
- As we move down the group in the periodic table, the size of the cations increases.
- Na+ is smaller than K+, which means Na+ can be more effectively surrounded by water molecules (hydration).
- Therefore, Na2Cr2O7 is actually more soluble than K2Cr2O7, making Statement A false.
- A primary standard must have a stable composition and not absorb moisture from the air.
- Na2Cr2O7 is hygroscopic, meaning it absorbs moisture, which affects its weight and concentration.
- Thus, Na2Cr2O7 cannot be considered a primary standard, making Statement B false.
- The chromate ion (CrO4^2-) has a central chromium atom surrounded by four oxygen atoms.
- The hybridization of Cr in CrO4^2- is sp3, leading to a tetrahedral geometry.
- Therefore, Statement C is true.
- The dichromate ion (Cr2O7^2-) consists of two chromium atoms connected by oxygen atoms.
- The structure indeed includes Cr-O-Cr bonds.
- Thus, Statement D is true.
- The false statements are A and B.
The false statements are:
- A: Na2Cr2O7 is less soluble than K2Cr2O7.
- B: Na2Cr2O7 is a primary standard in volumetry.
Topper's Solved these Questions
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS EXERCISE - Additional Problems for Self practice (APSP)
30 Videos
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS EXERCISE - National Standard Examination In chemistry
35 Videos
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS EXERCISE - Exercise 2
40 Videos
- CHEMICAL EQUILIBRIUMBOOK - RESONANCE ENGLISHCHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - Advanced Level Problems (Part-3)(Stage-5)
2 Videos
- GASEOUS STATEBOOK - RESONANCE ENGLISHCHAPTER - GASEOUS STATEEXERCISE - ORGANIC CHEMISTRY(Hydrocarbon)
17 Videos
Similar Questions
The maximum oxidation state shown by V(Z=23),Cr(Z=24),Co(Z=27),Sc(Z=21), are respectively
View SolutionThe aqueous solution of transition metal salt changes colour from pink to blue, when concentrated hydrochloric acid is added to it. The changes in colour is due to:
View SolutionMatch the compounds of column X with oxidation state of central atom in column Y.
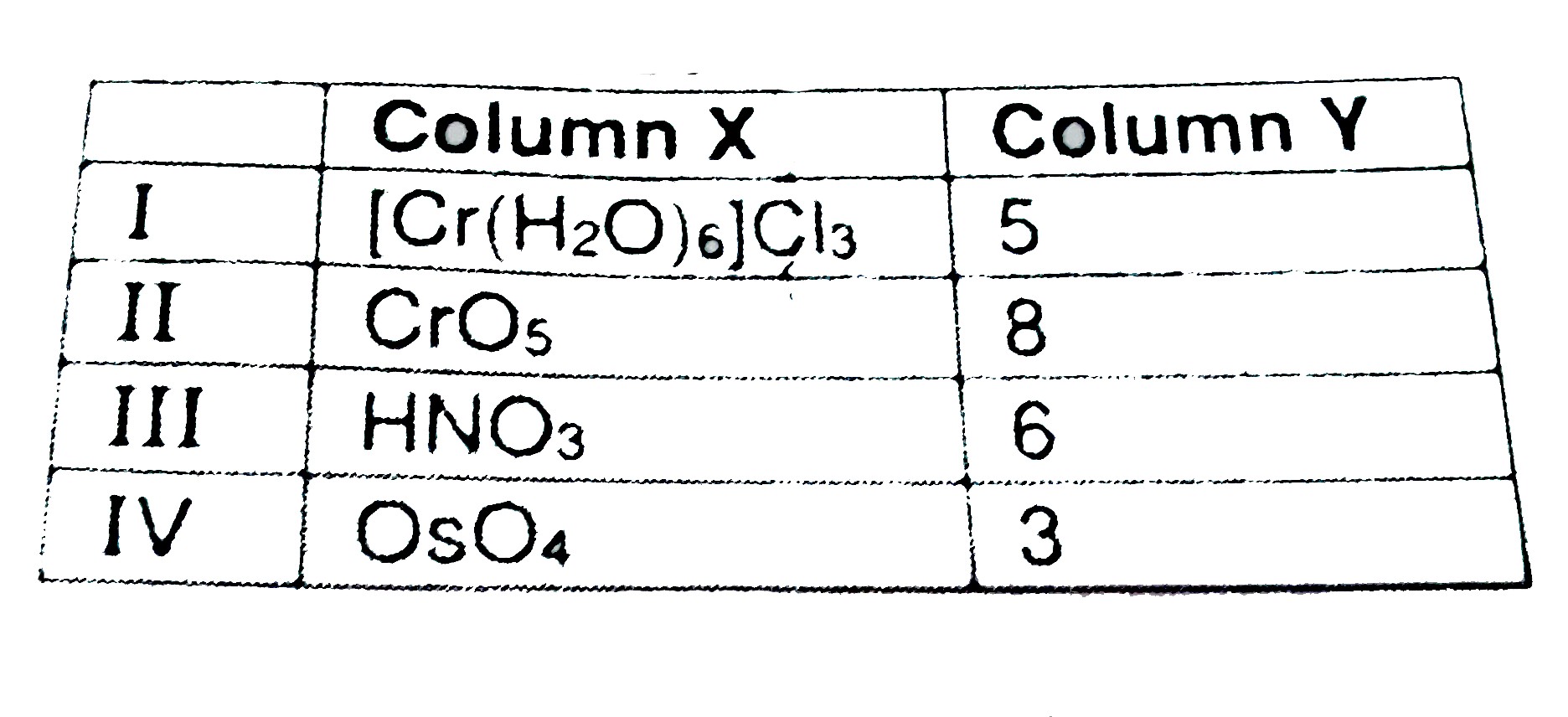 .
.
View SolutionAn alloy consists of rubidium and one of the other alkali metals. A sample of 4.6g of the alloy when allowed to react with water, liberates 2.241dm3 of hydrogen at STP.
Relative atomic masses: AT(Li)=7,AT(Na)=23,AT(K)=39,AT(Rb)=85.5,AT(Cs)=1.33
Which alkali metal is the component of the alloy?
View SolutionA certain quantity of a gas occupied 100ml when collected over water at 15∘C and 750mm pressure . It occupies 91.9ml in dry state at NTP. Find the V.P. of water at 15∘C
View SolutionWhich of the following statement is false ?
View SolutionWhich of the following statements are false ?
View SolutionWhich of the following statements are false.
View SolutionWhich of the following statements is false?
View SolutionWhich of the following statements is FALSE ?
View SolutionWhich of the following statements is false ?
View SolutionWhich of the following statement is false ?
View Solutionनिम्न में से कौनसा कथन असत्य है
View Solutionनिम्नलिखित में से कौन सा कथन गलत है
View Solutionनिम्न में से कौन-सा कथन असत्य है?
View Solutionनिम्न में से कौन-सा कथन असत्य है?
View Solution

