In the given reaction : CH3−C∣PhH−OH∣CH−CH3conc.H2SO4−−−−−−−→Δ Alkenes
Total number of alkenes (including stereo isomers) formed will be
To solve the question regarding the total number of alkenes formed in the given dehydration reaction, we will follow these steps:
Step 1: Identify the reactant
The reactant is given as:
CH3−CH(Ph)−CH(OH)−CH3
This compound has a hydroxyl group (OH) that will undergo dehydration.
Hint: Look for functional groups that can undergo elimination reactions.
Step 2: Understand the dehydration mechanism
The dehydration reaction involves the protonation of the hydroxyl group, leading to the formation of a carbocation. In this case, the hydroxyl group will first be protonated by sulfuric acid (conc. H₂SO₄), forming a better leaving group (water).
Hint: Remember that dehydration reactions typically involve the formation of carbocations.
Step 3: Formation of the carbocation
Upon protonation, the compound becomes:
CH3−CH(Ph)−CH2OH+2
This leads to the loss of water, resulting in the formation of a carbocation:
CH3−CH(Ph)−CH+2
Hint: Identify the carbocation formed after the loss of water.
Step 4: Carbocation rearrangement
The initial carbocation can rearrange to form a more stable carbocation through a hydride shift:
CH3−CH(Ph)−CH+2→CH3−CH(Ph)−CH2−CH+3
Hint: Consider the stability of carbocations and possible rearrangements.
Step 5: Determine possible alkene products
From the first carbocation, we can form two alkenes by losing a proton (H⁺):
1. From CH3−CH(Ph)−CH+2:
- Loss of H from the carbon adjacent to the Ph group gives:
CH2=CH(Ph)−CH3
- Loss of H from the terminal carbon gives:
CH3−CH(Ph)−CH=CH2
2. From the rearranged carbocation:
- Loss of H from the carbon adjacent to the Ph group gives:
CH3−CH(Ph)−CH=CH2
- Loss of H from the terminal carbon gives:
CH2=CH(Ph)−CH3
Hint: Consider all possible combinations of proton loss from each carbocation.
Step 6: Count stereoisomers
For the alkenes formed:
- The first alkene CH2=CH(Ph)−CH3 has no stereocenters.
- The second alkene CH3−CH(Ph)−CH=CH2 has a chiral center and can exhibit geometric isomerism (cis/trans).
Thus, we have:
- 2 geometric isomers from CH3−CH(Ph)−CH=CH2 (cis and trans).
- 1 from CH2=CH(Ph)−CH3.
Step 7: Total count of alkenes
Adding them up:
- From the first carbocation: 2 alkenes (1 with geometric isomers)
- From the rearranged carbocation: 2 alkenes (1 with geometric isomers)
Total:
- 2 (from first) + 2 (from rearranged) = 4 alkenes
- Including stereoisomers, we have 4 + 2 (from the chiral center) = 6 alkenes.
Final Answer: The total number of alkenes formed, including stereoisomers, is 6.
The reactant is given as:
CH3−CH(Ph)−CH(OH)−CH3
This compound has a hydroxyl group (OH) that will undergo dehydration.
The dehydration reaction involves the protonation of the hydroxyl group, leading to the formation of a carbocation. In this case, the hydroxyl group will first be protonated by sulfuric acid (conc. H₂SO₄), forming a better leaving group (water).
Upon protonation, the compound becomes:
CH3−CH(Ph)−CH2OH+2
This leads to the loss of water, resulting in the formation of a carbocation:
CH3−CH(Ph)−CH+2
The initial carbocation can rearrange to form a more stable carbocation through a hydride shift:
CH3−CH(Ph)−CH+2→CH3−CH(Ph)−CH2−CH+3
From the first carbocation, we can form two alkenes by losing a proton (H⁺):
1. From CH3−CH(Ph)−CH+2:
- Loss of H from the carbon adjacent to the Ph group gives:
CH2=CH(Ph)−CH3
- Loss of H from the terminal carbon gives:
CH3−CH(Ph)−CH=CH2
- Loss of H from the carbon adjacent to the Ph group gives:
CH3−CH(Ph)−CH=CH2
- Loss of H from the terminal carbon gives:
CH2=CH(Ph)−CH3
For the alkenes formed:
- The first alkene CH2=CH(Ph)−CH3 has no stereocenters.
- The second alkene CH3−CH(Ph)−CH=CH2 has a chiral center and can exhibit geometric isomerism (cis/trans).
- 2 geometric isomers from CH3−CH(Ph)−CH=CH2 (cis and trans).
- 1 from CH2=CH(Ph)−CH3.
Adding them up:
- From the first carbocation: 2 alkenes (1 with geometric isomers)
- From the rearranged carbocation: 2 alkenes (1 with geometric isomers)
- 2 (from first) + 2 (from rearranged) = 4 alkenes
- Including stereoisomers, we have 4 + 2 (from the chiral center) = 6 alkenes.
Topper's Solved these Questions
- ORGANIC REACTION MECHANISMS-IVBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS-IVEXERCISE - Exercise-2 Part-3
10 Videos
- ORGANIC REACTION MECHANISMS-IVBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS-IVEXERCISE - Exercise-2 Part-4
8 Videos
- ORGANIC REACTION MECHANISMS-IVBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS-IVEXERCISE - Exercise-2 Part-1
11 Videos
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - APSP Part - 3
22 Videos
- PERIODIC TABLE & PERIODICITYBOOK - RESONANCE ENGLISHCHAPTER - PERIODIC TABLE & PERIODICITYEXERCISE - ORGANIC CHEMISTRY(BASIC CONCEPTS)
24 Videos
Similar Questions
Predict the products expected in the given reaction
2-Bromo-1,1-dimethylcycopentaneC2H5OH−−−−→Δ
View SolutionAlcohols undergo acid catalysed elimination reactions to produce alkenes. Because water is lost in the elimination , this reaction is called dehydration reaction. Secondary and tertiary alcohols always give E1 reaction in dehydration. Primary alcohols whose β-carbon is branched also give E1 reaction. The reactivity of alcohol for elimination reaction is tertiary alcohol > secondary alcohol > Primary alcohol.
Which of the following dehydration product (major ) is incorrect ?
View SolutionThe relative rate of acid catalysed dehydration of following alcohols would be
I.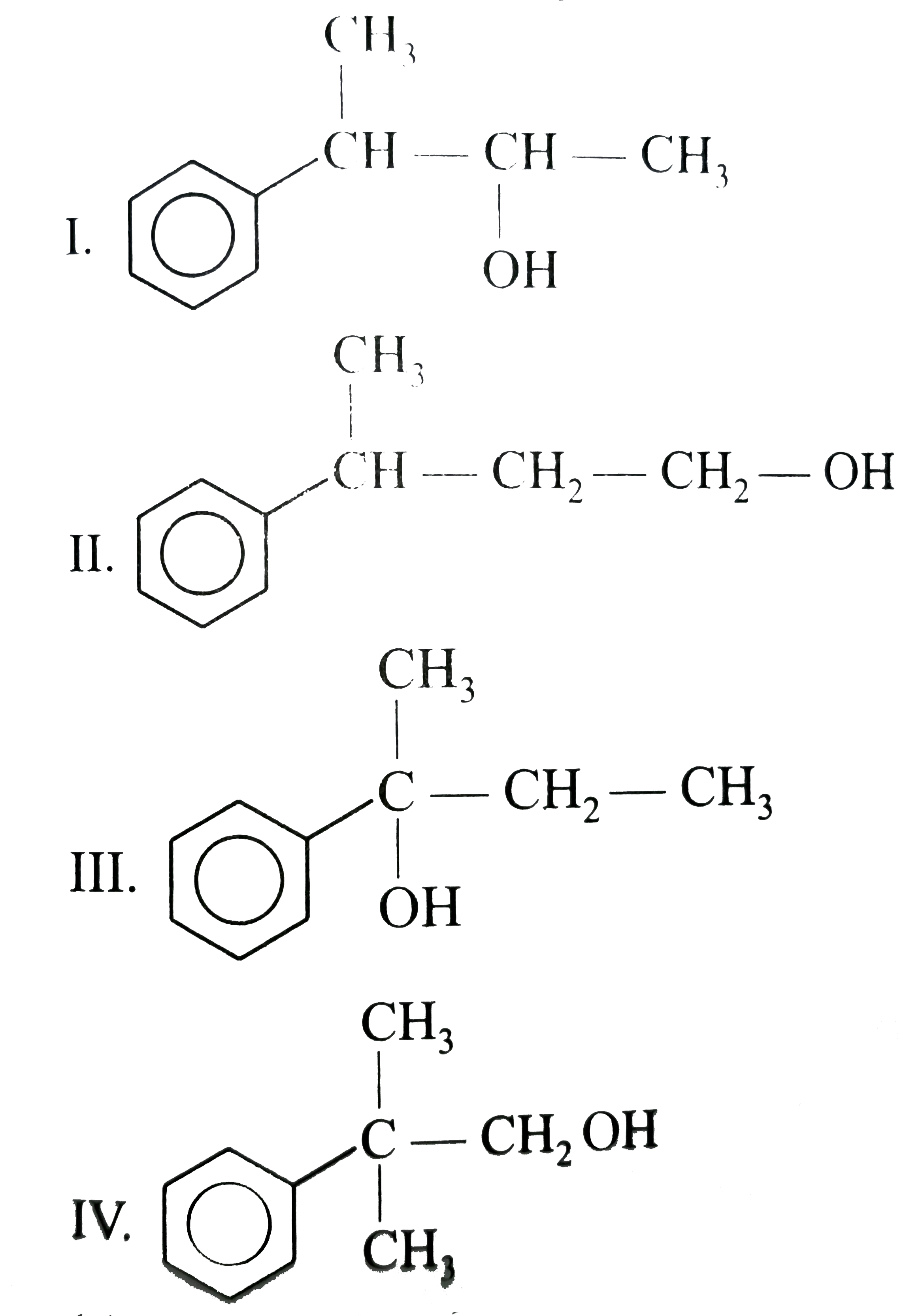
View Solution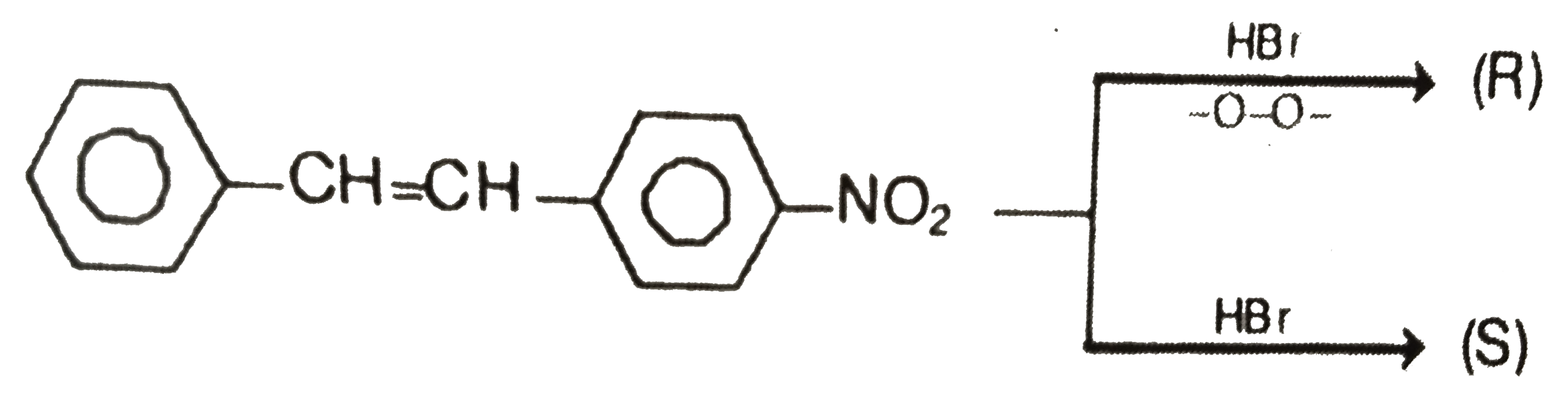
R & S are :
View SolutionAn element with atomic number 107 has recently been discovered. Its block, group number, period and outshell electronic configuration respectively are :
View SolutionCH3−CH3∣C∣H−CH2BrAlKOH−−−−→Δ Alkene,
Alkene is -
View SolutionCH3−C∣CH3H−Cl∣CH−CH3t−BuO−−−−→Δ Major Product is :
View SolutionIn the given reaction : CH_3-underset(Ph)underset(|)CH-oversetoverset(OH)(|)CH-CH_3underset(Delta)overset(conc. H_2SO_4)to Alkenes Total number of alkenes (including stereo isomers) formed will be
View SolutionCH3−CH3∣C∣CH3−CH2CH2CH2OHH+−−→
Alkene The alkene formed in the above reaction will be
View SolutionIn the given reaction CH3−OH∣CH−OH∣C∣CH3−CH3HIO4−−→a+b and B respectively be
View SolutionThe posible number of stereisomers of the product of following reaction would be :
Ph−CH=CH−CH∣CH3−C∣H=OH2N−OH−−−−−−→Ph=4.5+
View SolutionWrite the IUPAC name of the CH3−C∣CH3H−C∣OHH−CH3∣C∣CH3−CH3
View SolutionHow many stereo isomers are possible for
CH3CH=CH−C∣ClH−CH2Br
View SolutionCH2=CH−CH=C∣CH3−CH3+HBr−−−→CH3−Br∣CH−CH=C∣CH3−CH3+CH3−CH(II)=CH−Br∣C∣CH3−CH3
Correct statement about the above reaction is
View SolutionCH3−CH3∣C∣H−CH2BrAlKOH−−−−→Δ Alkene,
Alkene is -
View Solution

