Which of the following metal carbonates liberate. CO2(g) on heating :
Na2CO3
K2CO3
Rb2CO3
Ag2CO3
The correct Answer is:D
To determine which metal carbonates liberate carbon dioxide gas (CO₂) upon heating, we can follow these steps:
Step 1: Identify the metal carbonates in the options
First, we need to identify the metal carbonates provided in the question. Common metal carbonates include those of sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and silver (Ag).
Step 2: Understand the decomposition of metal carbonates
When heated, not all metal carbonates decompose to release carbon dioxide. Generally, the carbonates of alkali metals (like sodium, potassium, rubidium, and cesium) do not decompose upon heating. However, some transition metal carbonates, such as silver carbonate, do decompose.
Step 3: Analyze the behavior of each carbonate
- Sodium carbonate (Na₂CO₃): Does not decompose on heating.
- Potassium carbonate (K₂CO₃): Does not decompose on heating.
- Rubidium carbonate (Rb₂CO₃): Does not decompose on heating.
- Cesium carbonate (Cs₂CO₃): Does not decompose on heating.
- Silver carbonate (Ag₂CO₃): Decomposes upon heating to form silver oxide (Ag₂O) and releases carbon dioxide (CO₂).
Step 4: Conclude the results
From the analysis, we can conclude that among the given options, only silver carbonate (Ag₂CO₃) liberates carbon dioxide gas upon heating.
Final Answer:
The metal carbonate that liberates CO₂ on heating is Silver Carbonate (Ag₂CO₃).
---
First, we need to identify the metal carbonates provided in the question. Common metal carbonates include those of sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and silver (Ag).
When heated, not all metal carbonates decompose to release carbon dioxide. Generally, the carbonates of alkali metals (like sodium, potassium, rubidium, and cesium) do not decompose upon heating. However, some transition metal carbonates, such as silver carbonate, do decompose.
- Sodium carbonate (Na₂CO₃): Does not decompose on heating.
- Potassium carbonate (K₂CO₃): Does not decompose on heating.
- Rubidium carbonate (Rb₂CO₃): Does not decompose on heating.
- Cesium carbonate (Cs₂CO₃): Does not decompose on heating.
- Silver carbonate (Ag₂CO₃): Decomposes upon heating to form silver oxide (Ag₂O) and releases carbon dioxide (CO₂).
From the analysis, we can conclude that among the given options, only silver carbonate (Ag₂CO₃) liberates carbon dioxide gas upon heating.
The metal carbonate that liberates CO₂ on heating is Silver Carbonate (Ag₂CO₃).
Topper's Solved these Questions
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Exercise-2
Explore 44 Videos
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Exercise-3
Explore 10 Videos
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Matching List Type
Explore 1 Video
- QUALITATIVE ANALYSISBook:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSISExercise:INORGANIC CHMISTRY(Qualitative analysis)
Explore 35 Videos
- QUALITATIVE ANALYSIS PART 1Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS PART 1Exercise:A.L.P
Explore 37 Videos
Similar Questions
Knowledge Check
Which of the following compound will not liberate CO2 on reaction with NaOH?
A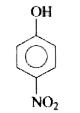 B
B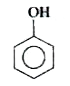 C
C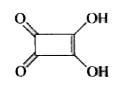 D
D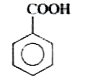
Which of the following metal carbonates is decomposed on heating?
AMgCO3BNa2CO3CK2CO3DPb2CO3Which of the following metals does not liberate hydrogen from acids?
AFeBCuCMgDZn
Out of the following which does not liberate N2 gas on heating?
View SolutionWhich of the following reaction liberates maximum amount of heat energy?
View SolutionWhich of the following does not have a metal carbon bond?
View SolutionWhich of the following will release CO2 when heated to 1000∘C?
View SolutionWhich of the following will liberate CO2 on reaction with NaHCO3 ?
View Solution
